What raises and lowers individual pool chemistry factors?

When troubleshooting a swimming pool, it helps to know what can cause individual chemistry factors to increase and decrease. This article is an overview of the most common chemistries we measure in swimming pools, and what causes their levels to change up or down.
We have also included a Rule Your Pool Podcast episode for each factor we talk about in this article.
Covered in this article:
What raises and lowers...
- pH
- Total Alkalinity (TA)
- Calcium Hardness (CH)
- Cyanuric Acid (CYA)
- Total Dissolved Solids (TDS)
- Combined Chlorine (CC)
- Phosphates
- Conclusion
What raises and lowers pH?
Quick facts
- pH is an equilibrium that moves constantly. It cannot be controlled, and it is not something measured in ppm (mg/L).
- Just about every chemical and contaminant has some impact on pH, though maybe not noticeable.
- With carbonate alkalinity in the water (every swimming pool), the pH is determined by the amount of CO2 dissolved in the water.
pH is an equilibrium of Hydrogen concentration:
pH = -log[H+]
In other words, pH tells us how acidic or basic the water is. The higher the concentration of Hydrogen in the water, the lower the pH and the more acidic the water is. The lower the concentration of Hydrogen, the higher the pH and the more alkaline (aka basic) the water is. pH is important because it impacts just about every other type of chemistry we deal with in swimming pools.
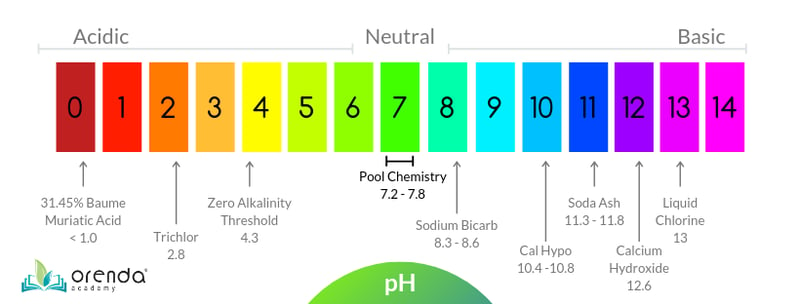
Every chemical you add to a pool has an impact on pH. Some are minor and negligible, while others are major. Every whole number on the 0-14 pH scale is logarithmic, meaning each number is 10x greater or less than the next whole number. 6.0 pH is 10x more acidic than 7.0, but 100x more acidic than 8.0 (because its 10x10). Our chart below shows common pool chemicals across the pH spectrum.
Trichlor at 2.8 pH is quite acidic. It will lower the pH of your pool. Soda ash at 11.3-11.8 pH is very high. If you use it too fast, it will cloud up your water because it spikes the pH too rapidly. Want to guess the difference between 11.8 and 2.8 pH? Let's do some quick math:
11.8 - 2.8 = 9
109 = 1,000,000,000 (one billion)
Yes, trichlor is one billion times more acidic than soda ash. These chemicals are orders of magnitude different when it comes to their pH. It can be daunting to think about, but fortunately, there's an easier way to think about pH.
If you have carbonate alkalinity in your water, the pH is largely determined by the amount of dissolved carbon dioxide (CO2) in your water. The more dissolved CO2, the lower the pH. The less CO2, the higher the pH.
What increases pH?
To increase pH, we need to decrease the amount of dissolved CO2 in the water. We can do this by simply aerating the water. Think waterfalls, spillovers, spa jets, sprayer features, vanishing edges, or people splashing around in the pool. We can also chemically raise pH by adding high-pH things to the water, like sodium bicarbonate or sodium carbonate (soda ash). Another way is if calcium hydroxide is dissolved into the water from cement. This can actually be caused by acid, which forces the pH to rebound up.
One common myth is that liquid chlorine and cal hypo raise the pH of the water. That's only partially true. These chlorine types temporarily raise pH, but after chlorine (in its killing form HOCl) oxidizes or kills contaminants, its byproduct is hydrochloric acid (HCl), which neutralizes the high pH. So these chlorine types are actually pH-neutral after they do their job.
Finally, salt chlorine generators raise pH. The pH rises because Hydrogen gas (H2) bubbles off in the salt cell, which creates turbulence. Turbulence aerates the water and accelerates the loss of CO2, so the pH goes up.
What decreases pH?
To decrease pH, we must increase the amount of dissolved CO2 in the water. This can be done using a CO2 injector. Many commercial swimming pools use CO2 systems for this very purpose. But the most common way to reduce pH is with acid.
Wait. Acid does not contain CO2 in it. And if we know we must increase the amount of dissolved CO2 in the water to lower the pH, how does acid create more CO2?
The answer is found in carbonate alkalinity.
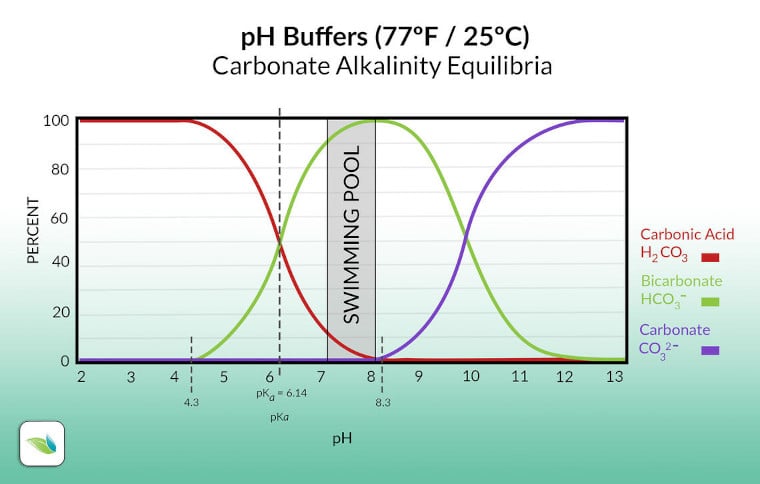
Most alkalinity in water is bicarbonate (green line). You can see in the chart above that the pH (x-axis) changes the percentage of bicarbonate ions in the water in equilibrium with carbonic acid (red line) and carbonate ion (purple line). If you look closely, you will see that carbonic acid is H2CO3. Let's look at that more closely:
H2O + CO2 ⇌ H2CO3
water + carbon dioxide ⇌ carbonic acid
In other words, carbonic acid is dissolved CO2. Adding acid converts alkalinity into carbonic acid–which increases the amount of dissolved CO2 in the water–and that lowers the pH.
So to lower pH, you need to either add CO2 directly to the water or add acidic chemicals to convert bicarbonate alkalinity into carbonic acid. Acidic chemicals include trichlor chlorine, citric/ascorbic acid, and other pool products. But muriatic acid or one of its alternatives is usually used to make a noticeable pH reduction.
What raises and lowers Total Alkalinity (TA)?
Quick facts
- Total Alkalinity (TA) is the sum of all dissolved alkali in the water, measured in ppm (mg/L).
- If TA increases, more alkali has been introduced to the water from somewhere.
- If TA decreases, either alkali has been removed (via dilution or Reverse Osmosis (RO) filtration) or it has been neutralized by acid or acidic chlorine (usually trichlor).
- Acid lowers pH and alkalinity simultaneously because acid converts bicarbonate into carbonic acid, which is dissolved CO2.
Total Alkalinity (TA) is the sum of all dissolved alkali in the water, measured in parts-per-million (mg/L). In swimming pools, most alkalinity will be made up of bicarbonate ions (HCO3-). If the pH gets over 8.3, your pool will get some carbonate ions too (CO32-). See the chart from earlier. There is also cyanurate alkalinity, which is approximately one-third of your CYA.1
The purpose of alkalinity is to be a buffer against changes in pH. Bicarbonate does this by both giving and taking a Hydrogen ion (H+). While alkalinity buffers against pH movement both up and down, it buffers better against reductions in pH, because bicarbonate neutralizes acids. When this occurs, acid adds Hydrogen to bicarbonate and converts it into carbonic acid (H2CO3). We just mentioned in the previous section that carbonic acid is dissolved CO2, and therefore both the pH and total alkalinity are reduced by acid.
What increases Total Alkalinity?
To increase TA, you must add alkali to the water. This can sometimes be done with high-alkalinity tap water, but it's almost always accomplished by adding sodium bicarbonate (baking soda) to the pool. You can also raise alkalinity by adding sodium carbonate (soda ash), but because it's ~1000x more basic than bicarb, you cannot use much of it. Sodium bicarbonate is the clear choice for raising TA in a swimming pool.
Another way that TA rises over time is if you're using a CO2 injector for pH management, coupled with a non-stabilized chlorine as your primary sanitizer. Because CO2 suppresses pH without reducing TA, the excess hydroxide from liquid chlorine and/or cal hypo accumulates over time. Normally, we never notice this because the acid easily neutralizes it. But when acid is not used, those hydroxides build up and contribute to TA.
What decreases Total Alkalinity?
Lowering alkalinity means reducing the amount of alkali in the water. Anything acidic enough to neutralize bicarbonate will accomplish this. In swimming pools, muriatic acid is the most common chemical used to reduce alkalinity. Muriatic alternatives like sulfuric and sodium bisulfate (dry acid) are also common. Another acidic chemical that neutralizes alkalinity is Trichlor.
Alkali can also be removed from the water, rather than just neutralized. Rain and snow dilution tends to lower alkalinity, and so does reverse osmosis filtration (RO). It's important to note that simply replacing water does not always mean alkalinity will be reduced. It depends entirely on the alkalinity level of the replacement water, and how the water was lost from the pool in the first place. If the water loss is from evaporation, the alkali in the water stayed in the pool (alkali like bicarbonate do not evaporate). But if your pool lost whole water (from a leak, long backwashing cycle, splash-out from bathers or overflow), then yes, your TA may be impacted on refill.
What raises and lowers Calcium Hardness (CH)?
Quick facts
- If calcium levels increase, the calcium came from somewhere. If you did not add a calcium product (like calcium chloride or cal hypo chlorine), it could have been from adding tap water with high calcium hardness. If not, the calcium is most likely being dissolved from the cementitious pool finish or tile grout due to an LSI violation.
- If calcium levels decrease, the calcium has gone somewhere. This means losing whole water (not just evaporation). Longer backwash cycles, intentional draining and diluting, or a pool leak are the most common.
- Reverse Osmosis filtration (RO) can also remove calcium from the water.
While the LSI tells us how balanced and saturated the water is with calcium carbonate (CaCO3), the amount of calcium carbonate is called calcium hardness (CH), which is measured with our test kits.
Calcium hardness is critically important for the overall balance of water. Water craves calcium and needs the right amount of it in given conditions (this is what LSI equilibrium is all about).
What increases calcium hardness?
If calcium hardness increases in water, the calcium came from somewhere. Either we added it, or the water stole it from cement (if available). The primary chemical used to raise CH is calcium chloride (CaCl2). Calcium can also be added by using calcium hypochlorite (cal hypo) chlorine. In 10,000 gallons of water, every pound of cal hypo adds about 4 ppm of CH to the pool.
If CH levels are going up but you did not add the calcium yourself, the water stole it from anywhere it could find it. That means dissolving calcium carbonate and/or calcium hydroxide from cement pool surfaces or tile grout. This is called etching, and it occurs when the LSI goes red on the Orenda Calculator™.
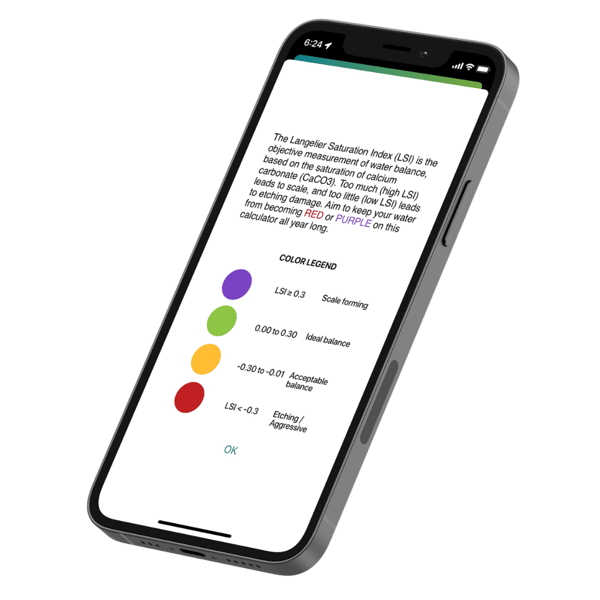
When the LSI is below -0.30, water is starving for calcium. It will stop at nothing to get it, even if your chemistry ranges are aligned with textbook recommendations! This is why we at Orenda prioritize LSI balance first before looking at range chemistry.
What decreases calcium hardness?
Reducing calcium hardness requires removing calcium from the water. This can be done via dilution or reverse osmosis filtration (RO). When diluting, like anything else, the replacement water must have lower calcium hardness than the whole water you remove from the swimming pool. Rain and snow are perfect examples of how to dilute calcium levels down.
Contrast that with people who have hard water out of their tap (say, over 300 ppm CH in the tap water). Reducing calcium hardness is more difficult because the replacement water has so much in it. That water is great for startups but not so good for diluting CH levels.
We can also reduce calcium hardness by forcing calcium carbonate out of solution. This can be messy and more labor intensive, but it also may be the only affordable option for pools in drought-stricken areas with water restrictions. Add a heavy dose of soda ash to the pool to force an LSI violation and precipitate calcium carbonate dust. Either filter out the cloudiness with a portable DE filter or shut off circulation and vacuum it out when the dust settles.
But dilution and RO filtration are the two best options for reducing calcium hardness.
What raises and lowers Cyanuric Acid (CYA)?
Quick facts
- If CYA levels increase, the CYA came from somewhere. If you did not add a CYA product (like granular or liquid cyanuric acid), or a stabilized chlorine (trichlor or dichlor), the test kit is likely inaccurate. Test with another CYA test to be sure.
- If CYA levels decrease, the CYA has gone somewhere or it has been oxidized over time. This means losing whole water (not just evaporation). Longer backwash cycles, intentional draining and diluting, or a pool leak are the most common.
- Reverse Osmosis filtration (RO) can also remove CYA from water.
Cyanuric Acid (CYA), aka stabilizer or conditioner, protects chlorine from sunlight. Without CYA, chlorine is destroyed rapidly by direct UV light. A little bit of CYA offers a lot of sunlight protection, but too much CYA is detrimental to pool chemistry in just about every way we can think of. So much so that we made minimal CYA our Fourth Pillar of proactive pool care.
What increases CYA?
You can add granular or liquid CYA directly into your water. This is usually done on commercial pools that use non-stabilized chlorines like liquid chlorine or cal hypo. The more common way CYA is introduced to water is through stabilized chlorines–trichlor and dichlor. Trichlor usually comes in 3-inch pressed tablets, and dichlor is usually sold as a granular chlorine shock. Both are about 50-55% cyanuric acid by weight. In 10,000 gallons of water, one pound of trichlor or dichlor will add 6-6.5 ppm of CYA. It accumulates quickly if you use trichlor every week.
What decreases CYA?
For residential swimming pools, this is the million-dollar question. Dilution and reverse osmosis filtration are the two best ways to reduce CYA. You can also stop adding more CYA (i.e. abandon using trichlor for several months) and allow for CYA to eventually be oxidized and break down in the water. But this takes months.
Some CYA removal products are on the market today. Customers tell us they have mixed results (at best). One is a nitrifying bacteria that breaks cyanuric acid down into uric acid, which eventually gets oxidized down into ammonia and chloramines. That's an oversimplification of chemistry, but trust us, the formulas are more complicated than we want to write.
We are hopeful that a safe, effective CYA removal product will exist one day. We think technology is trending that way, and it will be great when it arrives. For now, dilution or RO filtration are the best solutions.
What raises and lowers Total Dissolved Solids (TDS)?
Quick facts
- Most products leave some dissolved solids behind, so TDS tends to rise over time.
- TDS is the sum of all dissolved solids in water, primarily minerals, salt and metals, but also alkalinity and CYA.
- If TDS levels decrease, either the water was diluted or filtered with Reverse Osmosis filtration (RO).
Total Dissolved Solids (TDS) is the accumulation of all metals, minerals (including salt and calcium), cyanuric acid, alkalinity, and pretty much whatever else is dissolved in water.
What increases TDS?
Just about everything used in pools contributes to TDS is some way or another. The biggest culprit is salt. Salt can be added manually for a saltwater chlorine generator, or just by using liquid chlorine. In 10,000 gallons of water, one gallon of liquid chlorine leaves behind about 17 ppm of TDS (15 ppm of which is salt). Cal hypo chlorine also adds to TDS: in 10,000 gallons of water, one pound of cal hypo adds about 4 ppm of calcium hardness.
Anything that raises calcium hardness, total alkalinity, cyanuric acid or chlorine will add to TDS in some way. TDS can also accumulate from tap water replacing water that has evaporated out.
What decreases TDS?
Dilution and reverse osmosis filtration are the two best options for reducing TDS.
What raises and lowers Combined Chlorine (CC)?
Quick facts
- Combined chlorine is how much chlorine has combined with nitrogen compounds. It is measured by subtracting free available chlorine (FAC) from total available chlorine (TAC). The subtraction is simple: TAC - FAC = CC
- If there is any combined chlorine at all, there are (or were) nitrogen compounds in the water. There are no exceptions to this. So if combined chlorine levels rise, find the source of nitrogen and address it directly.
- If combined chlorine levels are decreasing, it's because it is being effectively oxidized and removed from water. This is a good thing.
- Reverse Osmosis filtration (RO) can also remove combined chlorine from the water.
Combined Chlorine (CC) is a measurement of chlorine that has bound to nitrogen compounds. It's part of the oxidation process to remove nitrogen from the water. Chlorine combines with nitrogen to eventually convert it into disinfection byproducts (DBPs) like chloramines.
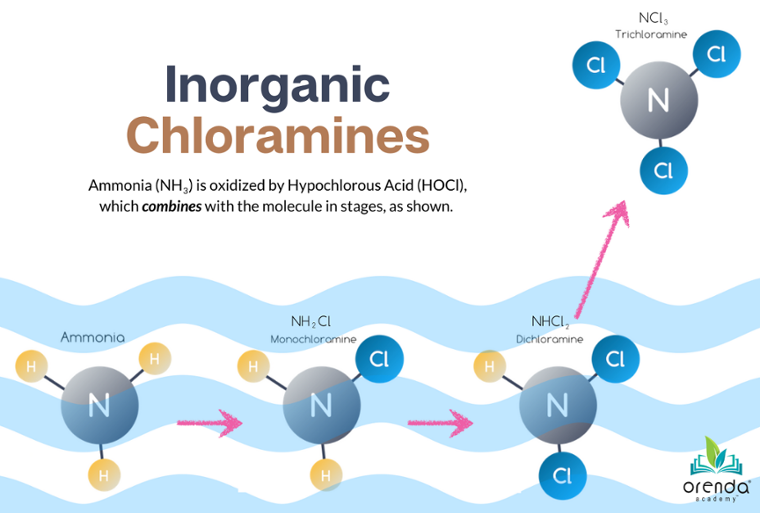
It's part of the breakpoint chlorination process:
Our chloramine and air quality website has several articles about this more in-depth topic. Combined chlorine also leads to indoor air quality problems and lung conditions like "lifeguard lung". So what contributes to combined chlorine?
What increases combined chlorine?
If you have combined chlorine, you have nitrogen in your water. It's the nitrogen compounds that increase combined chlorine. The more nitrogen, the more combined chlorine you will have. Look for anything that contains nitrogen being introduced to your pool.
Tap water is often chlorinated, but sometimes it is chloraminated, which means it's treated with monochloramine (NH2Cl) instead of chlorine. This is because monochloramine still has some disinfection power–albeit much slower and weaker than HOCl–but it lasts longer in the pipes.
The main sources of combined chlorine are urea from bathers peeing and sweating in the water, and ammonia-based products being introduced to the water. In commercial pools, look for ammonia-based deck cleaning products. In residential pools, the most common ammonia source is ammonia-based algaecides. Anything that contains words like "ammonium" or "quat" (short for quaternary ammonia) will lead to combined chlorine in your water.
What decreases combined chlorine?
For commercial swimming pools, this is the million-dollar question. Industry associations plead with swim teams to shower before swimming and stop peeing in the pool. But until the culture of competitive swimming changes, that is not a practical solution.
Combined chlorine is a worldwide problem, and disinfection/oxidation technology continues to advance to address it. Basically, you can reduce combined chlorine chemically and mechanically. You can also do reverse osmosis filtration (RO) and dilute water.
Chemically, you can use enzymes to break down the non-living organic components and help with the demand of chlorine in the water. Enzymes will not touch the nitrogen itself, but they can greatly simplify these compounds that chlorine must oxidize. Enzymes work very well with chlorine and facilitate the process of breakpoint chlorination.
Another chemical supplement to chlorine is non-chlorine shock, like potassium monopersulfate. This will oxidize precursors of combined chlorine, though it does not break down already-combined chlorine compounds.
Mechanically, secondary disinfection and oxidation systems are great for reducing combined chlorine. UV cannot oxidize anything, but it does have the capability to destroy monochloramines. Medium-pressure UV systems can also destroy dichloramine and trichloramine if they pass through the UV chamber. UV is great, but it's a point-of-contact system with no residual. Therefore it's at the mercy of circulation.
Secondary oxidizers like ozone and AOP are awesome at reducing combined chlorine. That is, assuming they are installed correctly and have good circulation. Like UV, ozone and AOP are point-of-contact systems. But unlike UV, these systems destroy combined chlorine and nitrogen compounds themselves.
Finally, you can filter out combined chlorine with reverse osmosis filtration, which removes almost everything in water. In our opinion, RO filtration is overkill for just combined chlorine. It would be used to remove more important things like CYA and accumulated minerals and metals.
What raises and lowers Phosphates?
Quick facts
- If phosphate levels increase, it's because phosphates are constantly being introduced to water through tap water, the environment around the pool, and/or phosphate-based pool chemicals.
- Phosphate levels can be decreased by using a phosphate remover (like PR-10,000) or by using Reverse Osmosis filtration (RO).
Phosphates are various forms of phosphorus found in water. There are several types of phosphates, but their building block is called an orthophosphate (PO4). Phosphate test kits usually only measure these orthophosphates. That's important to know because if you have condensed phosphate compounds in your water, the test kit may not identify them until they've broken down into orthophosphates.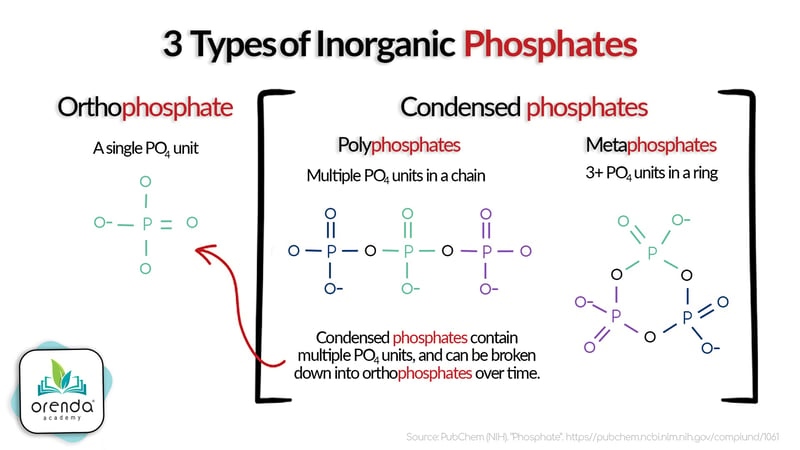
While phosphates are inert and do not directly contribute to chlorine demand, removing them is a great way to supplement chlorine and boost its efficiency. More on phosphates and chlorine demand here.
What increases phosphates?
Phosphates are introduced primarily 3 ways: chemically, environmentally, and through tap water.
Chemically, most sequestering agents for swimming pools are phosphate-based. These are products that help prevent metal stains and calcium scale.2 There can also be phosphate-based scale inhibitors in calcium hypochlorite (cal hypo) chlorine tabs and in some specialty acid products.
Environmentally, phosphates come from decaying organic materials like leaves, grass clippings, and soils. Lawn and garden fertilizers are heavily loaded with phosphates also. Another environmental source is bathers themselves. As a rule of thumb, plant and animal cells almost always contain some phosphorus, as it's an essential nutrient for life.
Tap water usually contains phosphates too. Ever since the Flint Michigan water crisis in 2014, municipal water treatment facilities across the country began to add phosphates to drinking water. Why? Because phosphate products can help protect the infrastructure from corrosion and scale. They can also bind metals and minerals into larger molecules for more efficient filtration and removal. Phosphates are a good thing to have in tap water because they protect us and they protect the infrastructure of the water grid.
Phosphates are also found in well water because water matriculates through the ground and down into the water table below. When in doubt, it's usually safe to assume your tap water contains at least some phosphates.
What decreases phosphates?
 We recommend keeping phosphate levels below 500 ppb (µg/L) in swimming pools. It's important enough that phosphate removal is our Third Pillar of proactive pool care. So how are phosphate levels reduced?
We recommend keeping phosphate levels below 500 ppb (µg/L) in swimming pools. It's important enough that phosphate removal is our Third Pillar of proactive pool care. So how are phosphate levels reduced?
You either need to use a phosphate remover like PR-10,000 or filter the water with reverse osmosis. You can also reduce phosphates if you dilute water and replace it with water that contains less phosphates in them (like rain and snow dilution).
Conclusion
Every chemical you add to water will impact the overall chemistry of the water. And some chemicals, like acid, impact multiple different chemistries simultaneously. The purpose of this article was to guide you when you have chemistry levels that go up or down. We have more in-depth articles on each factor that we linked to, and a podcast episode for each one too.
In the Orenda Program, we decide to remove as many variables from the water as we can. We simplify pool chemistry by obeying physics, focusing on LSI balance, and not over-chlorinating the water. Let this article be an outline for how to manage individual factors that may be giving you difficulty when you're trying to balance your water.
1 The exact amount of cyanurate alkalinity depends on both the CYA level and the pH of the water at that time. It is almost always between 30-33% in swimming pools, so the rule of thumb is one-third of CYA is your cyanurate alkalinity. This number must be subtracted from TA to get the corrected carbonate alkalinity when calculating the LSI. But the Orenda Calculator™ does this math for you. Our calculator even displays carbonate alkalinity as an optional secondary reading so you can see the impact pH and CYA have on it in real-time.
2 Our product SC-1000 is not a sequestering agent. It is a chelating agent, and it contains no phosphates. While it accomplishes the same tasks of inhibiting scale and preventing stains, chelation does so differently. Learn more here.