What is Alkalinity?

Total Alkalinity (TA) often gets confused with pH and words like alkali and alkaline. This topic is widely misunderstood in the pool industry. Most of us know how to test for TA, but may not know what it actually is, what it does, and why it's important.
Covered in this article:
- What is alkalinity?
- pH affects types of alkalinity present
- What does alkalinity do in water chemistry?
- Alkalinity buffers pH from changing
- Alkalinity and the LSI
- Ideal TA levels
- pH vs. Total Alkalinity
- Conclusion
- Further research
What is alkalinity?
Alkalinity is a measurement of dissolved alkaline substances in water (higher than 7.0 pH) that can neutralize acid. The three main types are:
- Bicarbonate (HCO3−)
- Carbonate (CO32-)
- Hydroxides
There are also other types of alkalinity that contribute to Total Alkalinity (TA), such as cyanurate alkalinity and borate. These other types need to be subtracted from TA to calculate the carbonate alkalinity, which is just bicarbonates and carbonates. More on that soon when we talk about alkalinity's impact on the LSI.
But without CYA and borate in water, alkalinity can be calculated using this formula (it's okay, you don't need to memorize this):
Alk = [HCO3−] + 2[CO32-] + [OH-] – [H+]
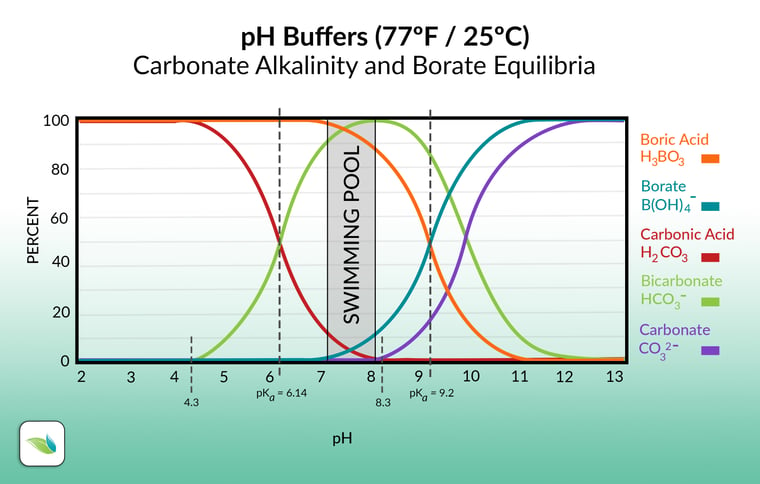
The pH affects the types of alkalinity present
The pH of the water determines which form of alkalinity is most prevalent in that moment. As you can see in the chart below, the different species of alkalinity exist in equilibrium with each other.
Let's move left to right on the graph. Carbonic acid is dissolved carbon dioxide (CO2). 4.3 pH is the point where alkalinity begins to exist in water. Anything below 4.3 pH, there is zero alkalinity in the water; just dissolved CO2. Essentially, at 4.3 pH, carbonic acid (the red line) begins to transition into bicarbonate (the green line). Pool chemistry should range between 7.2 and 8.2 pH, so it's almost all bicarbonate ions.
Then, at 8.3 pH, carbonate ions show up (the purple line), and the transition occurs from bicarbonate to carbonate ions. Notice the difference in all three of these are a Hydrogen ion. The higher the pH, the more Hydrogen dissociates and leaves. So carbonic acid (H2CO3) loses Hydrogen to become Bicarbonate (HCO3-). As the pH goes higher, at 8.3, bicarbonate loses its Hydrogen to become Carbonate (CO32-).
Here's the same chart, but only showing carbonate alkalinity:
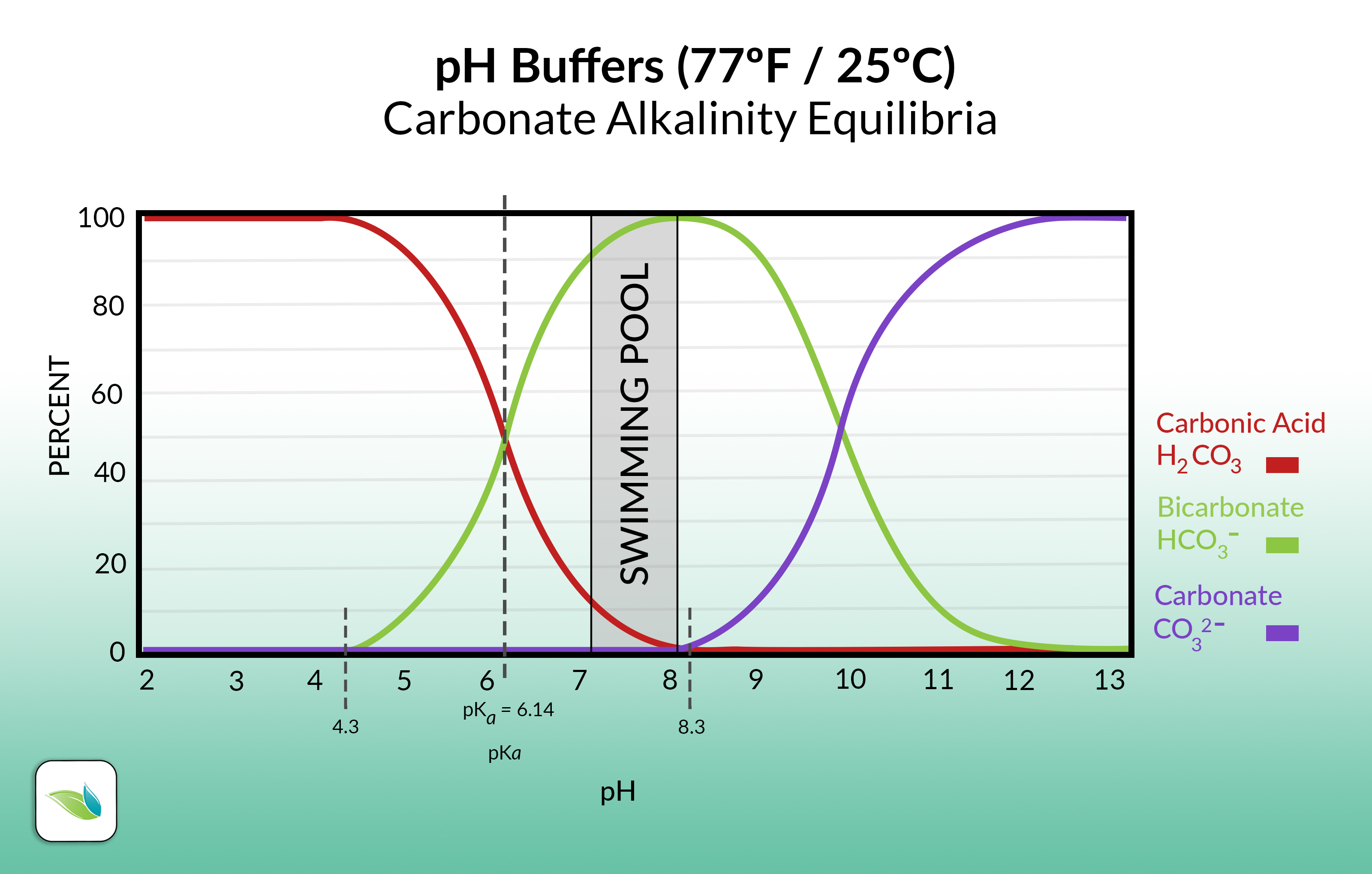
So as you can see, pH controls the species (and percentage of each species) of alkalinity present in water.
We included the boric acid and borate equilibria too, because if used, they contribute to total alkalinity but NOT carbonate alkalinity. Like cyanurates, borates need to be deducted from TA when calculating the LSI. More on that soon.
What does alkalinity do in pool chemistry?
Alkalinity buffers pH from changing
Alkalinity buffers (stabilizes) the pH of water by neutralizing acid. Since acid is defined by a high concentration of Hydrogen ions (H+), technically, alkalinity buffers pH by either providing or absorbing a Hydrogen ion as needed. So when acid is added, carbonate ions can absorb Hydrogen to create bicarbonate ions. This is an equilibrium, just like pH, so it can go the other way too. The equilibrium looks like this:
HCO3- + H+ ↔ H2CO3
Again, look back at the equilibrium chart to see the same thing. You'll notice the loss of Hydrogen as the pH goes higher.
Alkalinity and the LSI
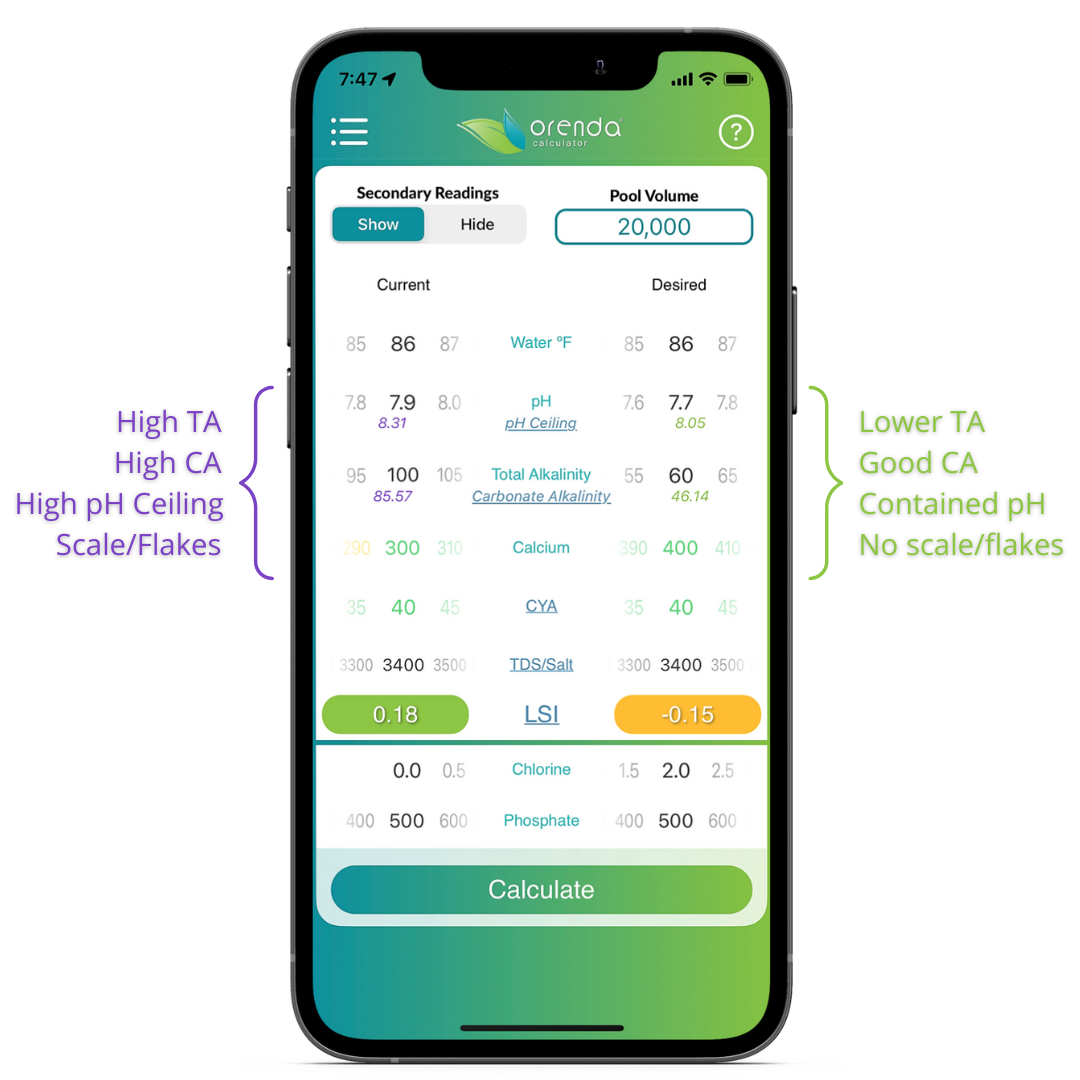
Carbonate alkalinity is a contributing factor to the Langelier Saturation Index (LSI). Use the Orenda Calculator™ to play around with different alkalinity levels to see how they affect the LSI. With Orenda 3.0, you can also opt to "show" secondary readings (top left toggle). This will show you how changing TA and other factors impact your carbonate alkalinity and pH ceiling.
You may notice you need to input your Total alkalinity (TA) on the calculator, because that's what your test kit will read. The other factors like CYA must be subtracted. Don't worry, the Orenda Calculator™ does all the math for you. Just put in your TA and the other factors, and the corrections are made automatically to calculate the LSI properly.
But just in case you want to know how carbonate alkalinity is calculated, here:
According to the IPSSA Basic Training manual, you can correct alkalinity by taking ~1/3 of the CYA level and subtracting it from the TA level. To be exact, you will need to know the pH of the water, but since pools are generally between 7.2 and 8.2 pH, you can get close with using 1/3. But again, the Orenda Calculator™ automatically makes this exact correction for you. Here's what the equation looks like, using a high CYA of 90 ppm and zero borates.
Corrected [carbonate] Alkalinity (CA) = TA ppm - (CYA ppm x [correction factor @ pH]) - (Borate ppm x [borate correction factor @ pH])
CA = 100 ppm - (90 ppm x [correction factor @ 7.6 pH])
CA = 100 ppm - (90 ppm x [0.33])
CA = 100 ppm - (30 ppm)
CA = 70 ppm
Ideal Total Alkalinity Levels
The ideal TA level depends on the type of sanitizer used, but textbook ranges generally recommend between 80-120ppm. We at Orenda disagree with this recommended range, especially in season. If your pool is cold and winterized, then sure, 80-120 is appropriate. But if you're using a salt chlorine generator, liquid chlorine, or cal hypo, you probably don't need so much alkalinity in your water.
We believe your TA should be a number that allows for a lower pH ceiling to limit your pH from reaching 8.3. You can use the Orenda Calculator™ to see your pH ceiling in real-time, as it is determined by your carbonate alkalinity. To offset this lower TA, you will need more calcium hardness to maintain overall LSI balance in the water.
Generally, we recommend having LSI-balanced water with 60 or 70 ppm TA, provided you are compensating for it with higher calcium levels AND you're not abusing acid. Always measure and dilute your acid before pouring it in.
Related: How to properly add acid to a swimming pool
What is the difference between pH and alkalinity?
We have an article that explains this more in-depth.
In short, pH is the power of Hydrogen (or potenz Hydrogen). It tells us the concentration of Hydrogen to determine how acidic or alkaline a substance is. It is an equilibrium that moves constantly and cannot be controlled. In swimming pools, thanks to carbonate alkalinity, the amount of CO2 dissolved in water largely determines the pH of the water. Knowing this helps us contain pH instead of trying (and failing) to control it.
Related: How to Lower pH and Alkalinity
Conclusion
Alkalinity is a measure of the substances that can grab those Hydrogen ions or give them away. This slows the transfer of Hydrogen ions (H+), and therefore alkalinity buffers against rapid changes in pH. Almost all the alkalinity in your water will be bicarbonate, but there are other types that factor into TA, including cyanurate alkalinity and borates. They need to be subtracted from TA when calculating carbonate alkalinity, which is one of the key factors in the Langelier Saturation Index.
Further research
To learn more, we encourage you to do research beyond this article. Some of our sources were:
- IPSSA Basic Training Manual, 2016 Revised Edition. By Robert W. Lowry
- The Importance of General Chemistry Relationships in Water Treatment. By Mealy & Bowman, Wisconsin DNR
- Study & Interpretation of the Chemical Characteristics of Natural Water. US Geological Survey
- Water Dissociation and pH. By Martin Chaplin
- Alkalinity and pH Relationships. By James McDonald, PE, CWT
- Startup Certification Course, National Plasterers Council. By Greg Garrett