LSI Balance and Calcium Management | Pillar 1

LSI Balance and Calcium Management is the first of Orenda's Four Pillars. This is arguably the most important of the Four Pillars because it is about understanding water and how it behaves. This article aims to simplify chemistry, so it can be more easily understood. If you are a chemist (who is not already familiar with this subject) and you would like to get more in depth on the science of this topic, this is one of our primary sources. We will link to other external sources in this article as well. So let's get into it.
Action Step: Adjust your water chemistry into LSI Balance each and every time you treat the water.
What is "Range Chemistry"?
Textbooks and classes teach ideal "ranges" for water chemistry. Example: "Alkalinity should be between 80-120 ppm" or "Calcium hardness should be between 200-400 ppm." Ranges are fine if the other factors of the LSI are allowing for balanced water...but oftentimes they are not. For example, the ranges are thrown off when you have a salt pool with some CYA in it. Or when the water temperature gets cold enough.
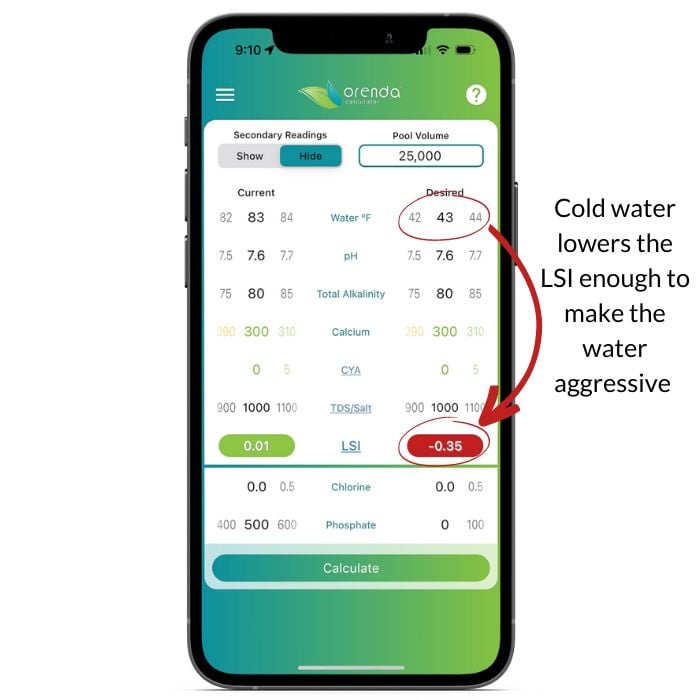
On the left, we have water chemistry set within the "ideal ranges", according to the textbooks. Just lowering the water temperature by 40ºF makes otherwise balanced water become aggressive.
LSI First, Range Chemistry Second
At Orenda, we believe you should focus on the LSI as the first priority, and try to align your ranges accordingly. A salt pool needs to be maintained differently than a cal hypo pool. And a winterized pool needs to be maintained differently according to the season and water temperature. Range chemistry, as a blanket objective, can lead to problems. So be sure your chemistry fits in accordance with the LSI.
We believe a solid water chemistry foundation is built with calcium hardness, which allows us more options for our alkalinity levels to contain pH. So let's talk more about calcium...
Calcium Carbonate Saturation
Water balance, in the context of swimming pools and other water systems, is about the saturation of calcium carbonate (CaCO3). You see, water wants equilibrium (ideal saturation) of calcium carbonate. We can measure the saturation of calcium carbonate on the Langelier Saturation Index (LSI); a formula originally created by Dr. Wilfred Langelier for closed-water systems1. The LSI has been adapted for swimming pools by factoring in an alkalinity correction for cyanuric acid. Now let's get back to the concept of equilibrium.
The perfect amount of calcium carbonate saturation would be 0.00 on the LSI. The "ideal range" is between -0.30 to +0.30, or according to some sources, as high as +0.50. Either way, there's a cushion of ideal saturation around 0.00 on the LSI. Water always wants to be in equilibrium. If it is undersaturated (low LSI), it will dissolve calcium anywhere it can find it until the water is balanced (0.00 on the LSI). If the water is oversaturated with calcium, it will deposit calcium carbonate in the form of scale, or carbonate dust.
There are six factors that determine the solubility of calcium carbonate...meaning they impact the LSI. The factors are:
- Water Temperature
- pH
- Carbonate Alkalinity*
- Calcium Hardness
- Cyanuric Acid*
- Total Dissolved Solids (TDS)
*Cyanurate alkalinity is factored out of Total Alkalinity in the LSI formula to calculate the Carbonate Alkalinity.
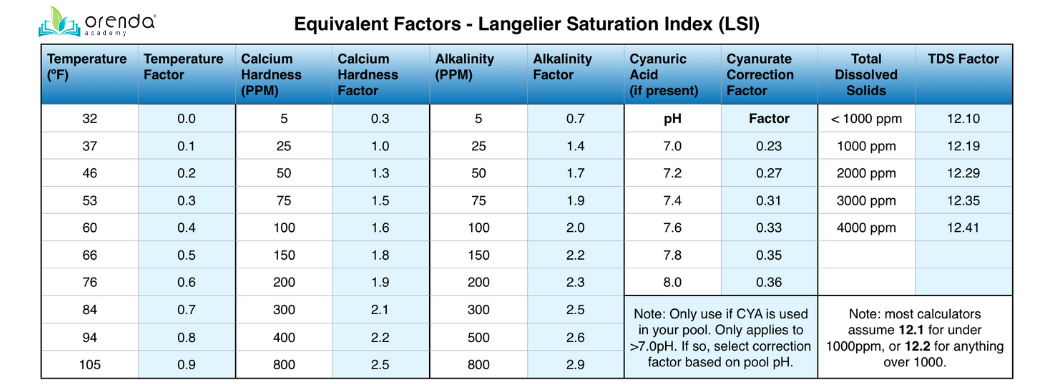
These variables are given numerical equivalent “factors”, as assigned in The Langelier Numerical Equivalents Table. See the chart below. The formula looks like this:
(pH) + (Temperature [ºF] Factor) + (Calcium Hardness Factor) + [(Total Alkalinity Factor) - (CYA correction factor @ current pH)] - (TDS factor) = LSI
Here are the LSI factors on a reference chart:
Don't worry, the Orenda App has all of these factors in place, so you just need to input the chemistry you test, and the app will do all the math for you.
An analogy to better understand LSI
When you add sugar to water or tea, you can stir it in until it dissolves. You can add more sugar and dissolve it too. At some point, however, when too much sugar is added, what happens to that sugar? No matter how hard you stir it, it just will not dissolve. The excess sugar will continue to swirl around until it settles out in the bottom of the glass, like the picture here shows.
That said, the amount of sugar that can be dissolved changes dramatically when you heat, the water. Boiling water can dissolve a lot more sugar than cold water. This is because temperature (and other factors) can change the saturation limit of water. Cool, huh?
Now replace "sugar" with "calcium", and we're talking about the LSI. The difference is with calcium, the colder the water, the more can be dissolved. It's the opposite of most substances, like sugar. This is why cold water is more aggressive: calcium carbonate is more soluble in colder water.
Facts about calcium equilibrium
Here's a cool thing about water: it cannot over-saturate itself. Meaning water cannot dissolve more calcium than it can hold in solution. Water cannot take itself over 0.00 on the LSI. If other factors change, like temperature rising, the water will naturally seek to lower itself back down to equilibrium by precipitating out calcium carbonate (which first looks like cloudy water, and eventually scale or calcium dust).
If you are in the pool business, you are probably all-too-familiar with these conditions. If you start up new pools with a cementitious surface (like pebble, plaster, quartz, etc.), when the pool is full, chances are the pH is over 8.0. Usually way over 8.0, right? There is a reason for that. The water found equilibrium on its own, by dissolving highly-alkaline calcium hydroxide ([Ca(OH)2], which has a very high pH of 12.6) from the cement. The slight rise in calcium hardness and sharp rise of pH meant the water found equilibrium with a high pH and stopped dissolving calcium. And that's just one example...there are many, many more calcium-related problems faced by pool operators everywhere. And they are all explained by the LSI.
Over-saturation (high LSI)
As mentioned before, over-saturation is the result of factors driving the LSI over +0.30. These factors are usually pH and water temperature rising, or maybe too large of an increase in alkalinity or calcium hardness by the pool owner or operator. The result of over-saturation is calcium precipitation in the warmest places first. Remember, water temperature is a driving factor in the LSI! Carbonate scale forms in the hottest places first, like your heater, salt generator, a spillway or tile line that gets a lot of sunlight and heat. Scale does not form in the cold, dark bottom of the pool, because there are warmer places in the pool for calcium to fall out of solution.
Plaster dust is another common form of calcium fall-out, though it was originally formed due to a low LSI condition. Such a condition led to the water leeching calcium hydroxide, which spiked the pH near the surface, causing a local LSI violation on the high side, and boom! Plaster dust falls out of solution and lands on the surface. Here's a video that explains more:
Under-saturation (low LSI)
 Ahh, yes. Here's where the real problems come in. When water does not have enough calcium carbonate, it finds and takes it from anywhere it can. Low LSI issues include etching and pitting of cementitious surfaces and tile grout, corrosion of pool equipment and other problems. Basically, a low LSI can be explained simply: water is looking for calcium, and will stop at nothing to find it.
Ahh, yes. Here's where the real problems come in. When water does not have enough calcium carbonate, it finds and takes it from anywhere it can. Low LSI issues include etching and pitting of cementitious surfaces and tile grout, corrosion of pool equipment and other problems. Basically, a low LSI can be explained simply: water is looking for calcium, and will stop at nothing to find it.
Remember, water cannot over-saturate itself, so it will only etch until LSI equilibrium is met. That said, as other factors continue to change, so too does the LSI. For example, if your climate is cold and your pools are winterized, as the temperature continues to drop, the LSI continues to get more and more aggressive. The lower the LSI, the more etching occurs to find equilibrium again. Play around with the temperature on the Orenda App and see it for yourself.
In cementitious surfaces like plaster or pebble, etching can often take pigment with it, leaving white marks or rings around aggregate, as shown in the picture. The rings indicate the loss of calcium, meaning at some point there was a low LSI violation severe enough to cause that damage. And yes, the damage is irreversible, even though it can be cleaned up and masked after-the-fact (like with an acid wash or re-polishing of the surface).
To avoid low LSI problems, use the Orenda App to ensure you have enough calcium hardness to handle the range of water temperature your pool will have. Build your foundation for water balance with calcium hardness because it does not change very much. Calcium is your best friend when it comes to water balance. Maintain the other factors of the LSI and keep the water in the ideal range year round.
Action steps: Managing Calcium and LSI
Because LSI and Calcium Management are the first pillar of proactive pool care, let's talk about how to do it. The objective here is simple: maintain ideal LSI year-round. You can use the Orenda App to dose your pool each visit, and it will show you what the current and desired LSI will be. The app simplifies a pool route and gives you control over the LSI balance of your pool. Knowing what the water wants–ideal calcium saturation–allows us to maintain control of our water chemistry. Here's a video on how to use the calculator:
We strongly recommend maintaining calcium hardness levels of at least 300 ppm. If your pool freezes, we suggest a minimum of 400 ppm. The Orenda LSI calculator will show you how to maintain even higher levels than that. You may be stunned to see how easy it can be to maintain a pool with 600+ ppm of calcium hardness. Why not build the foundation with calcium and make micro-adjustments to pH and alkalinity as needed? After all, you stand a much better chance of staying in LSI equilibrium year-round if you do.
And LSI equilibrium, friends, is Orenda's first pillar of proactive pool care.
1 Langelier, W. F. “THE ANALYTICAL CONTROL OF ANTI-CORROSION WATER TREATMENT.” Journal (American Water Works Association), vol. 28, no. 10, 1936, pp. 1500–1521. JSTOR, www.jstor.org/stable/41226418.