Evaporation and Accumulation

When water evaporates, it leaves behind minerals, metals, and other dissolved solids like salt. Naturally, whatever is left behind will accumulate over time. Let's discuss the impact that evaporation has on water chemistry, and how you can be proactive and adapt to it.
Water Evaporation
 Colorado River above the Hoover Dam. Source: Shutterstock
Colorado River above the Hoover Dam. Source: Shutterstock
Evaporation is the process in which water is converted into water vapor and released into the air. Water vapor is also called moisture, and the saturation level of moisture in the air is called humidity. There is absolute humidity, which is the total amount of moisture in the air, and there is relative humidity (RH), which is the amount of moisture in the air relative to how much the air can hold at a given temperature. These humidity terms are not important for outdoor swimming pools, because a single swimming pool is not going to make a noticeable difference to the overall humidity of the great outdoors. Relative Humidity in indoor pools, however, is very important, because it impacts the evaporation rate and many other variables pertaining to indoor air quality.
From a water chemistry perspective, we care most about the accumulation of substances that are left behind when water evaporates.
Outdoor swimming pool evaporation loss
Sources online indicate that an average outdoor swimming pool during the summer can evaporate between 0.25-0.50 inches (0.6 - 1.2 cm) per day. That means a swimming pool could lose up to 3.5 inches of water per week! And because evaporation is directly correlated to the surface area of water, this water loss would be equivalent no matter how big the pool is. Here is a video from an Orenda customer demonstrating a bucket test, showing water will evaporate from a pool at the same rate as a bucket. If the pool's water level is lower than the bucket's, there's obviously a leak.
In hotter climates like Arizona or Florida, evaporation is even faster. That said, because the humidity in Florida and the Gulf of Mexico is significantly higher than that of the dry Arizona desert, Arizona pools will evaporate faster at the same temperature. In other words, even with the same air and water temperature, dryer climates will evaporate pools faster than humid ones, because there is less moisture already in the air.
Indoor swimming pool evaporation loss
According to ASHRAE, swimming pool evaporation can be calculated using some complex formulas. Let's leave those formulas and calculations to professional engineers. Instead, we spoke with indoor pool dehumidification experts and asked him about indoor pool evaporation. As it turns out, an indoor swimming pool in a properly-dehumidified natatorium will evaporate approximately its entire volume of water in a year. That's a lot of water! Just imagine what your outdoor pool could evaporate in a year.
Mineral and Chemical Accumulation in Water
 When water evaporates, only H2O will go.1 Everything else, like the dissolved solids calcium, metals, and salt, as well as chemicals like cyanuric acid (CYA) and nitrates are left behind.2 Because these substances stay behind, their levels will accumulate over time. It should be noted that these accumulating factors happen in slightly different ways.
When water evaporates, only H2O will go.1 Everything else, like the dissolved solids calcium, metals, and salt, as well as chemicals like cyanuric acid (CYA) and nitrates are left behind.2 Because these substances stay behind, their levels will accumulate over time. It should be noted that these accumulating factors happen in slightly different ways.
-
 CYA will accumulate only with additional CYA being added, like when using stabilized chlorine. Evaporation compounds the issue, but source water (tap water) should not contain any CYA. So while the accumulation of CYA can be a big problem, evaporation is not the main cause of it. The main culprit is trichlor, which is 55% CYA by weight. One pound of trichlor in 10,000 gallons raises CYA by 6 ppm. It adds up fast.
CYA will accumulate only with additional CYA being added, like when using stabilized chlorine. Evaporation compounds the issue, but source water (tap water) should not contain any CYA. So while the accumulation of CYA can be a big problem, evaporation is not the main cause of it. The main culprit is trichlor, which is 55% CYA by weight. One pound of trichlor in 10,000 gallons raises CYA by 6 ppm. It adds up fast.
- Consequences: Over-stabilization, lower LSI (aggressive water), and in extreme cases, purple staining.
- Related articles:
- Calcium accumulates over time with evaporation because most tap water has at least some calcium hardness in it. Around the United States, we have found the average tap water–in our experience–is generally below 100 ppm. That's really low, especially for a pool startup. But it is enough that tap water will gradually add to the total calcium hardness level of the pool via refilling.
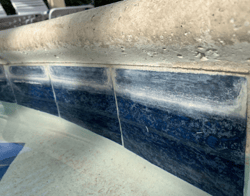 Calcium also accumulates in pools that use Calcium Hypochlorite (cal hypo) chlorine. If only using cal hypo as a granular shock, this rise in calcium is hardly noticeable. But if cal hypo is your primary chlorine, the rise is something that must be accounted for. According to the IPSSA Basic Training Manual, one pound of cal hypo will raise calcium hardness by 8 ppm in 10,000 gallons of water.
Calcium also accumulates in pools that use Calcium Hypochlorite (cal hypo) chlorine. If only using cal hypo as a granular shock, this rise in calcium is hardly noticeable. But if cal hypo is your primary chlorine, the rise is something that must be accounted for. According to the IPSSA Basic Training Manual, one pound of cal hypo will raise calcium hardness by 8 ppm in 10,000 gallons of water.
- Consequences: Raised LSI (leading to an over-saturation of CaCO3), carbonate scale formation.
- Related Articles:
- Metals are often in tap water (especially well water), and also get into pools from metal-based pool chemicals. Such chemicals include metal/mineral sanitizer systems and copper-based algaecides.
- Consequences: Increased chlorine demand, water discoloration, staining.
- Related Articles:
- Nitrates accumulate because they are the most reduced form of nitrogen found in water. In other words, they cannot be oxidized further by chlorine. Nitrogen compounds are present in many forms in swimming pools. Mainly, urea and ammonia. These compounds get oxidized by (and combine with) chlorine, as shown in the breakpoint chlorination process. Once these compounds are oxidized as far as they can be, you are left with nitrates in your water. Nitrates get into your water from bathers, animals, and the environment (soils, grass clippings, fertilizers, etc.).
- Consequences: Nitrates are a nutrient for algae, increased chlorine demand (from reproducing microorganisms), and combined chlorine.
- Related Articles:

- Salt and TDS accumulate when using liquid chlorine (sodium hypochlorite). Again, according to the IPSSA Basic Training Manual, one gallon of 12% sodium hypochlorite will leave behind up to 30 ppm of TDS. And according to our most-cited water chemistry expert, Richard Falk, 10 ppm of FAC from liquid chlorine leaves behind 17 ppm of salt. And while you might think saltwater chlorine-generator pools would have a salt accumulation problem, they usually do not. Their salt levels stay relatively stable unless more salt continues to be added.
- Consequences: Higher TDS, lower LSI (aggressive water), accelerated corrosion of metal equipment, cloudy pool water, possible link to calcium crystals (hypothesis only).
- Related Articles:
Solution by Dilution

You can be proactive about evaporation and chemical accumulation first by minimizing chemicals that leave behind long-term byproducts. The Orenda Program uses no algaecides or stabilized chlorine. Too many of these byproducts can render your water too contaminated to drain in states like California, so be aware of them!
You can also minimize evaporation by using a solid pool cover. Covers are especially beneficial for indoor pools, from an energy perspective. Outdoor pools can benefit too.
Now let's assume you have too much of something in your water already. Reduction can be accomplished through draining and diluting, or specific filtration.3 Basically, if the pool's fill water does not contain (or has a low level of) the substance you are trying to reduce, dilution will help. Dilution is the best, cheapest and safest solution to:
- Remove/reduce cyanuric acid (CYA)
- Remove/reduce calcium hardness
- Remove/reduce nitrates
- Remove/reduce salt and TDS
- Remove/reduce metals
Draining half your pool is a great way to cut your CYA and TDS levels approximately by half. But draining and refilling is not going to necessarily lower your metal levels, because most pools with metal issues have metal in their tap water. An exception to this would be pools using a mineral sanitizer system that dissolves metals into the water intentionally.
Metal removal is often more practical with specific metal-removing filters.
Dilution does not always have to come from draining a big portion of the pool, however. Extended backwash times can help discard water and dilute with fresh water. Rain can help dilute chemistry down, especially calcium hardness and TDS, because rain is pure water with no minerals or dissolved solids in it.
Finally, pools that leak also lose water, and while leaks are definitely a problem, we bring it up because some people may not be aware they have a leak. We have had a few customers of ours learn they had a leak because they could not figure out why calcium and CYA levels were dropping over time. If they were not draining the water themselves, water was escaping on its own.
Conclusion
As water evaporates (up to a few inches per week), minerals and substances like CYA are left behind. The pool will be refilled periodically, and the chemistry of the tap water will add to whatever is in the pool. Tap water contributes to calcium and heavy metal accumulation especially. It should not contribute to CYA or salt accumulation, because neither substance should be in tap water.
Pool chemicals like chlorine (liquid chlorine, cal hypo, and stabilized chlorines like dichlor and trichlor) all leave long-term byproducts behind. Liquid chlorine leaves behind salt and other TDS. Cal Hypo leaves behind calcium. And worst of all, stabilized chlorines leave behind CYA, and it accumulates quickly. Other chemicals, like algaecides, all leave behind long-term byproducts too.
This article is meant to inform you about what can accumulate and why. With the exception of metals, which can be filtered out with specialty filters, these accumulating substances are difficult to remove without draining water. At Orenda, we preach a minimalist approach to pool chemistry that is proactive, has no chemical conflicts, and leaves no long term byproducts behind. So if your pool has readings that are getting too high to handle, it's time to drain some water and get your chemistry back under control. Evaporation is guaranteed to happen, and we need to act accordingly.
1 There is an exception that we want to clarify here. In an indoor pool, some disinfection byproducts (DBPs) like Nitrogen Trichloride (aka Trichloramine) and Trihalomethanes like chloroform will off-gas along with evaporation. But these DBPs were never "dissolved" in the first place. These substances are chemical byproducts of chlorine oxidation, and technically do not evaporate; they off-gas. While water vapor rises and expands (like heat), DBPs that go airborne do not. They stay low in the natatorium just above the water surface. These DBPs are the root cause of indoor air quality problems in pools.
2 This means rain and snow have zero calcium hardness and TDS. Therefore, precipitation is very low on the LSI (hint hint, those of you who winterize pools).
3 An example of specific filtration would be a metal filter, or reverse osmosis (R.O.).
