Nitrates, Nitrites, and Urea in Swimming Pools

Nitrates, Nitrites and other Ammonia-based compounds can be confusing. We'll try to simplify it and why these things matter in swimming pools. We encourage you to read more into these subjects if you want to learn more.1
Covered in this article:
- What are nitrites and nitrates?
- What is urea?
- How to remove nitrites, nitrates and urea from swimming pools
-
- Breakpoint chlorination
- Specialized filtration methods
-
- Conclusion
What are Nitrites and Nitrates?
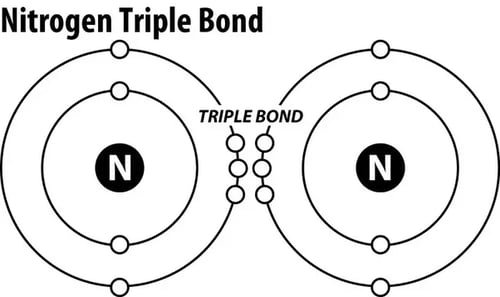
Nitrogen is an essential nutrient for all life. About 78% of our air is Nitrogen gas (and only about 19-20% Oxygen). It's all around us, and yet, Nitrogen gas (N2) is bonded together with a triple covalent bond, which renders it unusable to plants and animals.
Nitrates (NO3-) and Nitrites (NO2-), are usable forms of Nitrogen to plans and animals. Since Nitrogen gas (N2) has a strong bond between both Nitrogen atoms, it needs to be split and converted into different forms to be used by organisms. This splitting of N2 requires nitrifying bacteria and/or strong electrical process like lightning.
For plants, certain types of bacteria have an enzyme called nitrogenase, which can split the nitrogen bond and convert it into Ammonia (NH3), which then converts to Ammonium (NH4+) when it is mixed with water, which plants can use. These nitrifying bacteria can then convert Ammonia into Nitrite and Nitrate, which are even easier for plants to use than Ammonium. So that's how Nitrates and Nitrites are formed naturally.
This is all important because the EPA has limits for nitrites in drinking water.2 In chlorinated water, nitrites can be oxidized into nitrates.3
Just as there are different types of phosphates, there are two types of nitrates and nitrites: organic and inorganic. In swimming pools, we are primarily dealing with inorganic nitrates and nitrites.
How do Nitrites and Nitrates get into swimming pools?
Nitrites, Nitrates, and Ammonia are most commonly found in fertilizers, cleaning products, and even swimming pool algaecides.
Human waste contains nitrogen too. Urine makes up about 80% of the nitrogen excreted from humans in the form of urea. Urea directly ties into chloramines, pool air quality, disinfection byproducts, and many more water quality topics. We'll elaborate on urea in the next section.
The more ammonia in chlorinated water, the more prevalent nitrates will be. According to the US Geological Survey:
"Nitrate can also be formed in water bodies through the oxidation of other forms of nitrogen, including nitrite, ammonia, and organic nitrogen compounds such as amino acids."
What is Urea?
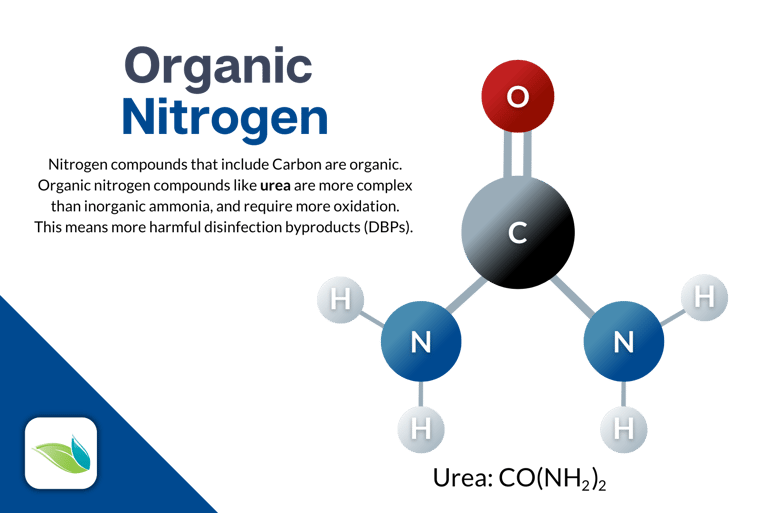 Urea (CH₄N₂O) is an organic form of nitrogen regularly introduced to swimming pools by bathers. Many swimmers pee in the pool. Urine contains uric acid and urea, which can then be broken down into Ammonium (mentioned earlier) and nitrate. The inorganic ammonia (NH3) undergoes some substitution reactions with chlorine, creating chloramines. These are also called combined chlorine.
Urea (CH₄N₂O) is an organic form of nitrogen regularly introduced to swimming pools by bathers. Many swimmers pee in the pool. Urine contains uric acid and urea, which can then be broken down into Ammonium (mentioned earlier) and nitrate. The inorganic ammonia (NH3) undergoes some substitution reactions with chlorine, creating chloramines. These are also called combined chlorine.
How does urea get into swimming pools?
Focusing on all the specific reactions and forms of nitrogen is beside the point. The point is that when swimmers pee in the pool, they contribute to the nitrogen loading. Nitrogen, in its various forms, is a nutrient for algae and other microorganisms. So we don't want it in our water.
We recognize that competitive swimmers will continue peeing in pools until there is a paradigm shift in swimming culture. Until that happens, urea in pools will continue to be an issue. Nitrites, nitrates, ammonium, and chloramine contamination will accompany it. Studies have been published online if you want to get more information about the health impact of all this.4
So what can we do about it?
How to remove nitrites, nitrates, and urea from swimming pools
Once these nitrogen compounds are in your water, it's not so simple to remove them. Thankfully, urea can be chemically managed, albeit at the expense of a lot of chlorine and oxidizing power. Nitrites get oxidized into nitrates, which leaves nitrates as the reduced form of nitrogen that cannot be oxidized out of the water. That means specialized filtration or dilution is required to remove nitrates from swimming pools.
Breakpoint chlorination
Let's start with how swimming pools can remove urea. Generally speaking, it needs to be oxidized. If your pool is fortunate enough to have a secondary oxidizer system like ozone or AOP, this is a much easier task than without such systems.
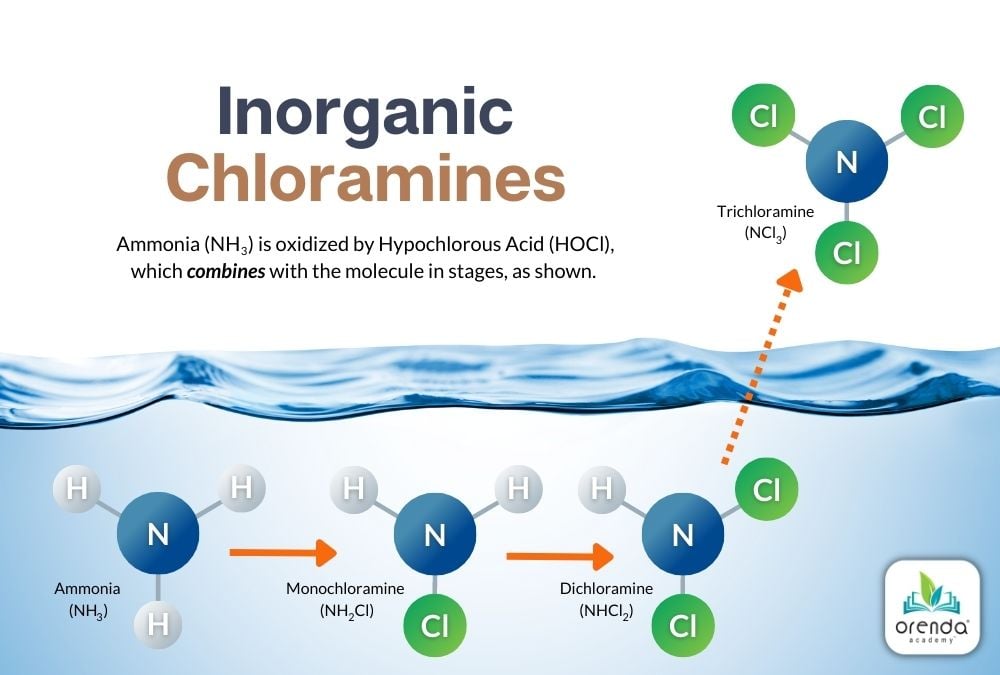
Urea is more complex than inorganic ammonia. Inorganic ammonia is relatively straightforward to oxidize into chloramines in three stages: mono-, di-, and tri-chloramine. Each at a 5:1 molar ratio of HOCl to NH3.
These chloramines are destroyed by overwhelming them with enough chlorine to complete the process illustrated above. This process is called breakpoint chlorination.
But urea is more complicated because it has carbon involved, making it both a nitrogen AND organic compound. It is therefore a much more complex oxidation process.5
But there's good news! Enzymes like CV-600 can simplify this considerably by addressing the carbon in urea. Enzymes will not break down nitrogen itself, but they help organic urea be broken down more easily into inorganic nitrogen, which goes through the abovementioned process. This can save a pool on chlorine consumption.
Our enzymes do not remove urea, nor do they remove nitrites or nitrates. They just break down carbon bonds, reducing the energy required for chlorine or secondary oxidizers.
As for nitrites and nitrates, chlorine oxidizes nitrite into nitrate2, but we're still left with nitrates, which cannot be further oxidized. And that leads us to specialized filtration methods.
Specialized filtration
According to the CDC:
"Nitrate may be successfully removed from water using treatment processes such as ion exchange, distillation, and reverse osmosis. Contact your local health department for recommended procedures.
Heating or boiling your water will not remove nitrate. Because some of the water will evaporate during the boiling process, the nitrate levels of water can actually increase slightly in concentration if the water is boiled. Mechanical filters or chemical disinfection, such as chlorination, DO NOT remove nitrate from water.
Remember to have your well water tested regularly, at least once a year, after installing a treatment system to make sure the problem is controlled."
So there you have it. Normal pool filters and chemicals cannot remove nitrates from the water, but specialized filtration, like reverse osmosis and ion exchange, can.
 R.O. filtration can be used on pools to remove almost everything from water.
R.O. filtration can be used on pools to remove almost everything from water.
If the cost of R.O. or ion exchange is prohibitive, draining and diluting your pool may be the best alternative.
Conclusion
Nitrogen compounds can be oxidized through the breakpoint chlorination process, and the chemistry is complex. We did not dive into it in this article. With urea, because it's an organic nitrogen compound, enzymes can help break down the carbon bonds and simplify the chemistry.
All nitrogen compounds eventually get oxidized down into nitrites and/or nitrates. Nitrates cannot be removed by filtration other than reverse osmosis (RO) and ion exchange. So if you have nitrates in your water, you're pretty much stuck with them unless you physically remove them or dilute water.
1 In our research for this subject, we read a lot of scientific papers online, and they go in further depth than we need to. We have simplified and summarized the information here. Our sources include the CDC for drinking water toxicity information, and you can find plenty of information online if you want to learn more.
2 US Environmental Protection Agency (2020). Use of Total Nitrate and Nitrite Analysis for Compliance Determinations with the Nitrate Maximum Contaminant Level. 40 CFR §141.23. EPA.gov
3 Yang, H., Cheng, H. (2007). Controlling nitrite levels in drinking water by chlorination and chloramination. Separation and Purification Technology. Vol. 56, issue 3 (p. 392-396)
4 Beech, J.A., Diaz, R., Ordaz, C., and Palomeque, B. (1980). Nitrates, chlorates, and trihalomethanes in swimming pool water. American Journal of Public Health. Issue 70 (p. 79-82).
5 Maybe one day we will expand on this and show you the exact chemistry, but we don't want carpal tunnel syndrome from typing all the formulas out, selecting the numbers for subscripts and superscripts, etc. It would take a lot of time and we'd still probably type something wrong.
