Proactive Pool Winterization: How to Prevent Calcium Crystals

Cold water is more aggressive than warm water. Not surprisingly, most pool surface damage occurs during the winter. Problems like calcium crystals and winter dust can be prevented by taking an LSI-based approach to pool winterization. In this article, we will explain how.
Covered in this article:
- Cold water and the LSI
- Water chemistry under the swimming pool safety cover
-
- Mesh pool safety covers
- Solid pool safety covers
- Pool winterization kits miss the real problem
-
- How to winterize a pool the Orenda way
- Adjust the LSI (water balance)
- Temperature swings
- Remove contaminants (water quality)
- Adjust the LSI (water balance)
- Conclusion
Cold water and the LSI
The Langelier Saturation Index (LSI) is the objective measure of water balance. One of the parameters factored into the equation is water temperature. The colder the water, the lower the LSI, and therefore most pool surface damage happens during the winter. And this is true regardless of pool type (cementitious, vinyl liner, or fiberglass).
Winter damage to pools
Cold, aggressive water is looking for calcium, and it will stop at nothing to find it. In a cement-based pool, calcium is in the surface itself, which is why cold, aggressive water etches cement. It is the result of water dissolving calcium compounds in the cement and pulling them into solution. This occurs because water needs that calcium to balance itself.
 This photo shows a pool finish with white halos around each piece of aggregate. This is from the loss of calcium hydroxide (Ca(OH)2) due to cold, aggressive water. The water was not LSI balanced in the winter, and therefore it became hungry for calcium, and this is the result. This is a common form of etching on cementitious surfaces.
This photo shows a pool finish with white halos around each piece of aggregate. This is from the loss of calcium hydroxide (Ca(OH)2) due to cold, aggressive water. The water was not LSI balanced in the winter, and therefore it became hungry for calcium, and this is the result. This is a common form of etching on cementitious surfaces.
In vinyl liner and fiberglass pools, calcium is not so available. This means cold, aggressive water stays starving for calcium for a prolonged period of time, and the damage can be ongoing until the water is able to restore LSI balance. Prolonged exposure to aggressive (low-LSI below -0.30) water leads to permanent surface damage.
In vinyl liners, this means loss of pigment (fading), as well as increased porosity in the vinyl material itself.1 Over time, this weakened liner is more susceptible to freeze/thaw cycles and expansion/contraction, which leads to wrinkling.
In fiberglass pools, cold, aggressive water degrades the gelcoat surface over time. Once compromised, chlorinated water can oxidize the polymers in the gelcoat, which turn white. This white hazing is often mistaken for scale, but it’s actually the opposite problem; it was caused by low-LSI water attacking the surface. We call this condition fiberglass chalking.

Now let’s talk about in-ground concrete pools with plaster-type surfaces.
Winter dust

Winter dust is a condition where water steals calcium from the cement-based pool finish as the water gets colder, then that calcium comes back out of solution as the water warms up in the spring. It’s a common LSI violation, especially on swimming pools that winterize with a solid safety cover. The sequence goes like this:
Water temperature drops > LSI gets lower > water gets hungry for calcium > water steals the calcium it needs > the dissolved calcium compounds increase both calcium hardness and the pH of the water > the water gets back to LSI balance and stops eating.
The water remains LSI balanced throughout the coldest months of the year, until…
Water temperature rises > LSI gets higher > water has too much calcium > water precipitates excess calcium carbonate (CaCO3) as dust > the precipitation lowers the calcium hardness > the water gets back to LSI balance and stops precipitating calcium dust.
Calcium (calcite) crystals

.jpg?width=1200&length=1200&name=Calcite%20crystals%20(flaky%20crystals).jpg)
.jpg?width=1200&length=1200&name=Calcite%20crystals%20underwater%20(2).jpg)



Just like winter dust, calcium crystals are also a form of calcium carbonate called calcite. We know of at least four types of calcite crystals. We have nicknamed them (as shown in the photo gallery above), and we outline each of the four types of crystals here.
In short, calcite crystals are a condition where water steals calcium slowly from the cement-based pool finish as the water gets colder. But instead of pulling it into solution completely, the process occurs gradually over many months in cold, stagnant water. The pH of calcium hydroxide in the cement is very high (12.6), and as this is dissolved, that high pH stays next to the surface, creating a high LSI violation locally.
Crazy, right? These crystals pull the high pH into the water and a little bit of calcium, but much of the calcium dissolved comes right back out of solution and builds small crystals on the surface.
Under a lab microscope, we can see hollow tunnels in each crystal, indicating calcium is being channeled out of the pool surface through the tunnel, much like magma comes up through a volcano.
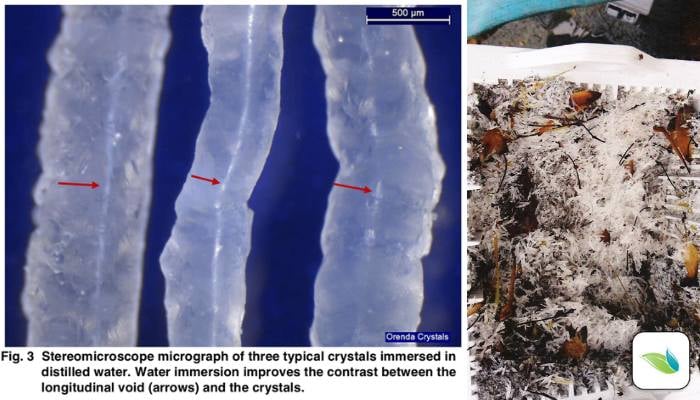
The calcium is then crystalized due to the high pH at the opening, and the crystal grows.
Water temperature drops > LSI gets lower > water gets hungry for calcium > water steals the calcium it needs > the high pH of the calcium compounds dissolved creates a high-LSI condition next to the surface, which crystalizes the calcium as it is drawn out of the cement > the crystal grows, and the water gets the remaining calcium and high pH to help it get back to LSI balance so it stops eating.
The water may not completely reach LSI balance if you have crystals in the winter, but it usually does when the water temperature warms up in the spring. By that time, the crystals already formed. We know crystals do not form in the spring because the lab tests indicate these crystals grow out of the surface.2
If these crystals formed in the spring when the water warmed up, they would be deposited as scale. Calcite crystals are NOT scale.
Water chemistry under the pool safety cover

The type of safety cover affects the pool chemistry during the winter, and therefore it affects the winterization process.
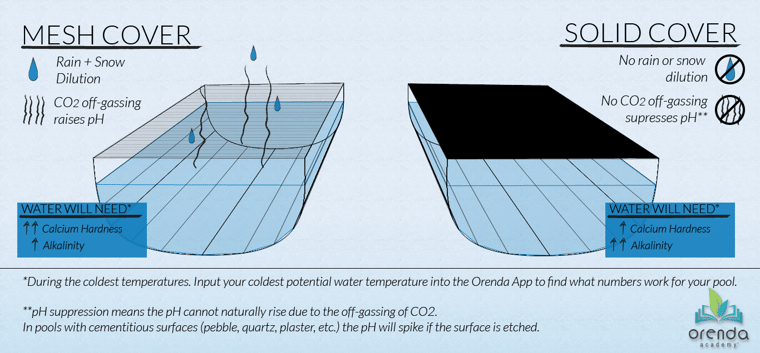
Mesh pool safety covers
Pools with a mesh cover (or no cover) are impacted by dilution and off-gassing CO2, as well as leaves and other organics that may sit on the cover for a while and drop tannins into the water.
We at Orenda view dilution and off-gassing CO2 as major advantages over solid covers. And to mitigate the tannins and other contaminants getting into the pool, just use a leaf blower and keep debris off the pool cover. It's that simple.
Rain and snow are both distilled water that contains no calcium, cyanuric acid (CYA), or TDS. So if your pool has accumulated any of these factors, rain and snow are your friends.
And the reason we like off-gassing CO2 is because the pH can naturally rise up to its pH ceiling and stay there all winter long. This means water is more predictable and better insulated throughout the winter months.
Pools with mesh covers need elevated calcium hardness to prepare for colder temperatures and dilution, along with a moderate amount of total alkalinity to get a desired pH ceiling.
Solid pool safety covers
Pools with a solid cover (or auto-cover) do not get the benefit of dilution or off-gassing CO2, but at least leaves and other debris will not get in.3 Because of the lack of dilution and natural pH rise to its ceiling, pools with solid covers may not need as much calcium as those with mesh covers, but they will need more total alkalinity. See the graphic above.
Pool winterization kits miss the real problem
Winterization kits contain a cocktail of pool chemicals that sound nice and beneficial, but they miss the point. Products in the winterization kits we have seen are focused on water quality, not water balance.
Winterization products usually include a chlorine shock of some kind, an algaecide–usually a polyquat 30, 60, or 90-day product, a metal sequestering agent, and maybe some other stuff. None of these address water balance.4
And furthermore, when the water gets cold enough, none of them work anyway. Chlorine lasts a few days at most, and the algaecide will contribute to chlorine demand. None of this is proactive.
The real issue is LSI balance. More specifically, calcium deficiency. If we were to summarize this entire article in two sentences: swimming pools need enough calcium hardness and alkalinity to maintain LSI balance during the coldest water temperatures of the year. Either we give water the LSI balance it craves, or it will destroy our pools in pursuit of calcium to restore that balance.
In the Orenda program, the winterization kit sounds more like this:
- Calcium chloride
- Sodium bicarbonate
- Non-stabilized chlorine
- CV-600 or CV-700 enzymes
- PR-10,000 phosphate remover
How to winterize a pool the Orenda way
There are two main water chemistry objectives in winterizing a swimming pool.
- Maintain LSI balance throughout the winter, and
- Remove contaminants so opening in the spring is easier.
Of course, there are other things that must be done, such as blowing out water lines and plugging them, winterizing equipment, and covering the pool. But let’s stay focused on water chemistry.
Related: Orenda Winterization Procedure
We all want to prevent a pool from turning green or brown during the winter. But unlike traditional pool care, our first priority is LSI balance. With that taken care of, preventing water quality issues is as simple as removing variables from the water before we put the cover on.
Adjust the LSI (water balance)
To prepare for the cold, water usually needs more calcium hardness. It also needs an alkalinity level that provides a favorable pH ceiling so that when the pH is at that ceiling (at the coldest water temperature), the LSI is balanced. This is easily done using the free Orenda Calculator™.
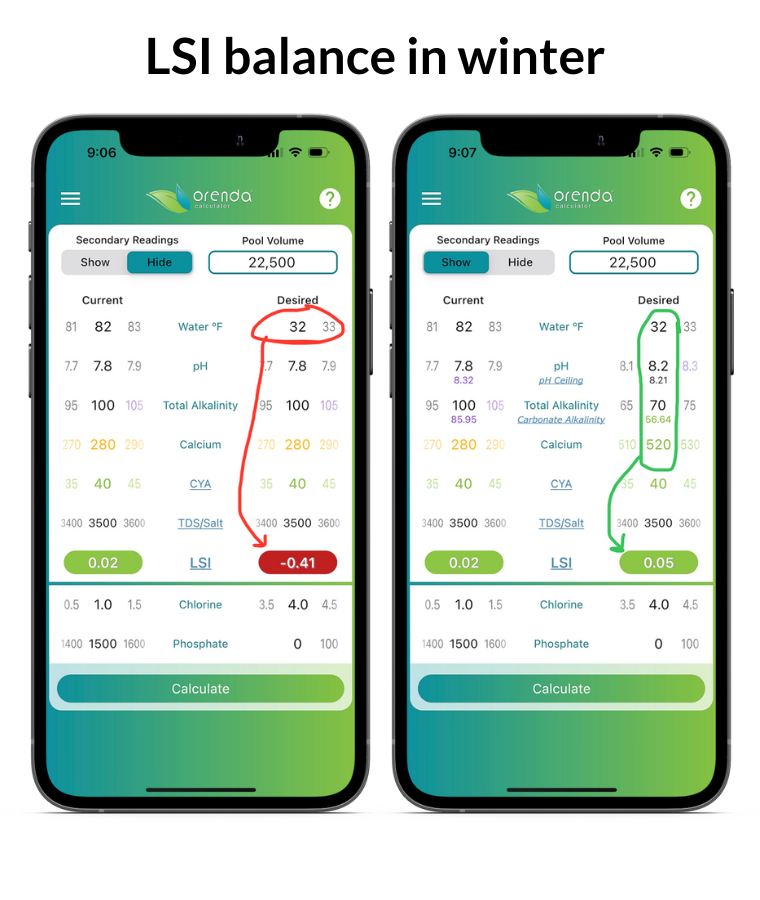
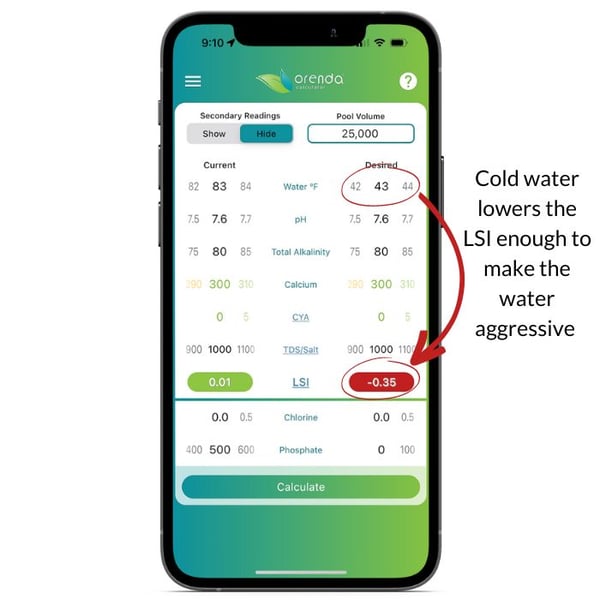
On the left, the Orenda Calculator™ demonstrates dropping the water temperature to freezing makes the water aggressive. On the right, we remedy this by increasing calcium and reducing total alkalinity so our pH ceiling is favorable. Follow these steps in order:
- Test your water chemistry and input it in the Orenda Calculator™. Then lower the water temperature on the right side of the calculator, and figure out how you want to get the LSI on the right to be green…something between 0.00 and +0.30.
- Pick a total alkalinity level that gives us a favorable pH ceiling (between 8.0 and 8.29 for winter)
- Find out how much calcium hardness your water will need when the temperature is at its coldest possible level (in many parts of the country, that's 32ºF/0ºC because water freezes). Rain and snow contain zero calcium and usually an acidic pH. This dilution will change your water chemistry if you have a mesh cover (or no cover). Because of this dilution and cold temperature, do not be afraid to add more calcium to your pool for the winter. The water will need it. 500-550 ppm or more calcium hardness is sometimes needed if your pool is likely to freeze.
- Once these parameters are set, pH will naturally rise to its ceiling and stay there during the winter (for pools with mesh covers or no covers). Pools with solid covers should have these parameters adjusted a few days before covering the pool to allow time for CO2 to off-gas and the pH to rise to its ceiling.
Important note: Do not add sodium bicarbonate and calcium chloride at the same time. You might be able to add them on opposite sides of the pool, but start with chelated calcium following this process, then slowly add the sodium bicarb on the other side of the pool. If it clouds up, slow down and dilute more.
Temperature Swings

Because swimming pools are closed in the fall, the water will be warmer than in the winter. One concern–and it's a valid one–is the risk of making the water scale-forming (> +0.30 LSI) before the temperature drops.
To account for this gradual decrease in water temperature, you can ease into winterization with multiple visits to the pool as the weather continues to cool. Consider a winter watch program if you are not already doing so. Multiple visits have benefits in themselves, as you can test the water and gauge progress.
If you are a homeowner reading this, just take your time adjusting your chemistry as the water gets colder.
On the flip side, when the water warms up, the LSI will rise gradually along with it. This is to be expected, and thanks to your proactive winterization approach in the fall, you have a few things working in your favor:
- the pool was most likely diluted by rain and snow over the winter,
- if it wasn't, the pool is about to be diluted when refilling the pool in the spring, and
- water will not go from the increase 50º overnight. We have time to ease out of winterization just like we eased into it. If the pool has a heater, do not crank it up too quickly. It's much easier to manage a 10-15º increase than a 40º-50º increase.
Remove contaminants (water quality)

Now we move over to the water quality side of the winterization process. It's simple: remove contaminants. We recommend removing non-living organics, oils, and other hydrocarbons with CV-600 or CV-700 enzymes and removing phosphates with PR-10,000.
Because PR-10,000 precipitates phosphate dust, give yourself a few days for the dust to settle or be filtered out so it can be removed before covering the pool. For a pool pro, this might be one week. For a homeowner, it could be a day or two.
If your pool has been maintaining an enzyme residual the entire season, that’s awesome! You have no need to add more enzymes when winterizing. If not, we suggest purging the pool before the water temperature gets below 75ºF/23.8ºC. Ideally, an Orenda pool gets an enzyme purge only once a year, in the spring when the water is warming up (65ºF/18.3ºC and rising).
PR-10,000 is a different animal than enzymes, and we recommend it for every winterization.
If you do these two things, simply chlorinate normally before putting the pool to bed for the winter. You should not need to superchlorinate.
Conclusion
LSI First, range chemistry second. Predict the cold and prepare for it, or else your pool will suffer the consequences. That is the proactive way to winterize a pool.
1 Fiberglass pools contain no calcium available in the material, but vinyl liners technically do have trace amounts of calcium carbonate in them. Yes, vinyl contains some calcium, and the loss of that calcium to aggressive water increases porosity in the material. This leads to wrinkling and pigment loss (fading).
2 We want to take a moment to thank the pool owners and service companies who have helped us learn about these crystals by providing us samples. We have sent several samples of crystals to the lab, and that is why we know of at least four types. They are all calcite, but they take on different shapes. They also vary in strength and stubbornness of removal. As of now, we still do not know why...but we do know that proactive winterization prevents crystals from coming back the next year.
3 There are some exceptions to this. Some solid pool safety covers have a type of hatch in the middle of the cover to allow for some water to drop into the pool, which helps prevent flooding on top of the cover. If you have such a cover, that's great news, because your pool will benefit from dilution. But most solid pool covers do not have this hatch.
4 It should be noted that a sequestering agent COULD keep calcium in suspension, but most people add these products when the water is already too cold, so it’s a moot point. And even if they did work, you would still need enough calcium hardness in the pool to begin with. Simply adding a sequestering or chelating agent does not change the fundamentals of the LSI...they only give some grace on how well calcium stays in solution/suspension.
