Understanding Heavy Metal Oxidation and Staining
This article discusses dissolved metals in swimming pool water, and how they impact water chemistry. For instance, when metals are oxidized, what do they do? We will cover oxidation, staining, sequestration and chelation, and more.
Covered in this article:
- What are heavy metals?
- Metals vs. minerals
- Heavy metal toxicity
- How to metals get into swimming pools?
- Metal oxidation and staining
- What is oxidation?
- Sequestering vs. Chelating
- Is sequestering or chelating right for my pool?
- How to prevent metal stains in pools
- How to remove metal stains in pools
- Conclusion
Before getting into this article, please understand that we are not subject-matter experts on metals and stains. We are here to simplify what we have learned through our research, but we encourage you to explore more information on your own.1
What are heavy metals?
In swimming pool chemistry, heavy metals are elements like iron, copper, manganese, and cobalt that can be present in water. These dissolved metals can be oxidized by chlorine (and other oxidizers), and at a high enough pH, they can come out of solution and stain pool surfaces. The two most common pool stains are oxidized iron and copper.
- Iron tends to turn orange/brown/red when oxidized.
- Copper tends to turn blue/green/turquoise when oxidized.
- Manganese tends to turn purple/black when oxidized.
- Cobalt turns blue when oxidized.
Other heavy metals sometimes found in drinking water include cadmium, arsenic, nickel, mercury, chromium, zinc, and lead.2 Hopefully, these metals are not in your pool or drinking in any measurable amount. If so, you should strongly consider a metal filter in your home or aquatic facility to remove them before human contact.
Metals vs. Minerals
The terms metals and minerals are often used interchangeably, so to reduce confusion, let's clarify what we mean. In swimming pool chemistry, metals refer to the types of metals that can be oxidized by chlorine, ozone, potassium monopersulfate, and other swimming pool oxidizers. Minerals are usually alkali earth metals like calcium and magnesium, which do not get oxidized, but can be carbonated.
Metals can be oxidized and stain pool surfaces.
Minerals can be carbonated and can precipitate as various forms of scale.
Water balance depends on having proper saturation of calcium carbonate, which we consider a mineral. Just like drinking water, minerals are not necessarily a bad thing to consume...unlike heavy metals. Nobody wants to drink water with copper, iron, lead, or other heavy metals in it. At certain concentrations, these metals are considered toxic.3
Heavy metal toxicity
Copper, for example, becomes toxic to fish at relatively low levels. For decades, copper sulfate was the algaecide of choice in ponds, but research has shown toxicity levels in fish are significant enough that it should not be used.4 But what about humans?
According to the EPA, the limit for copper concentrations is 1300 ppb, or 1.3 ppm. NSF International tests filters and other copper remediation systems in one of their drinking water standards, NSF/ANSI Standard 53.5 Regardless of the type of heavy metal in the water, at some point it will be considered toxic, though those levels vary. At Orenda, we prefer not to have them in our water in the first place.
How do metals get into swimming pools?
Metals can get into swimming pools in various ways, much like phosphates do. Metals are present in soils and rocks, and can be therefore introduced environmentally. Decorative flagstone around pools, for instance, should be sealed to help prevent rainwater from leaching iron from the stone and putting it into the pool.
There are also metal-based pool products that deliberately put metals into the water (think copper algaecide, silver chloride, copper ionizer systems, and other metal-based products). Most of these are chelated metals, but that nevertheless increases total metal levels in water.
Finally, tap water often contains metals; especially well water. In our experience with customers, tap water is usually the primary culprit or at least a contributing factor.
Other factors include galvanic corrosion of metals, or chemical corrosion (due to low pH and LSI) of copper components, such as pool heaters. Water is the universal solvent, and in the right conditions, it can dissolve just about anything.
Wherever your metals are coming from, we at Orenda have a three-point philosophy:
- proactive pool care, with
- no chemical conflicts, and
- no long-term byproducts left behind.
We can chelate metals with SC-1000, but they are still in the water. We prefer not to have metals in the water in the first place. Over time, metals accumulate, and at some point they become problematic.
Metal oxidation and staining



Metal stains in pools happen when enough dissolved metals are oxidized that they begin to precipitate. Metal staining usually happens faster at a higher pH. Much of what we know about stains comes from pool industry sources, which you can find easily online.
Since heavy metals can be oxidants, they contribute to the oxidant demand on chlorine. They are the first and easiest things for chlorine to oxidize. The energy needed for chlorine to oxidize iron, for instance, is lower than the energy needed to oxidize a more complex substance like sunscreen. And as oxidants, metals reduce chlorine when they are oxidized. This is why metals are shown in the breakpoint chlorination process:
Heavy metals themselves are virtually invisible when totally dissolved in water. That is, until those metals are oxidized. It is worth a quick recap of oxidation, in case you have not read about it already.
What is oxidation?
Oxidation is when an atom loses electrons. Oxidation usually occurs in an oxidation-reduction (or “redox”) reaction. All that means is an oxidizer (like chlorine's active form, HOCl, in swimming pools) steals electrons from the atom. Reduction is actually the opposite of oxidation. When an oxidizer like chlorine steals electrons from the oxidant, those negatively-charged electrons (e-) reduce the charge of the oxidizer. It eventually breaks down HOCl into useless chloride ions that can no longer oxidize (Cl-).
Related: Understanding Oxidation-Reduction Potential (ORP)
Example: Iron is oxidized by chlorine, water, and oxygen itself
Let’s take the easiest example: Iron (Fe). Iron is an easily oxidized metal because it does not hold its electrons with a very strong bond. When something like Oxygen (O2), Water (H2O) or Hypochlorous acid (HOCl) meets it, Iron is the reducing agent, because it loses electrons.6 Chlorine is the oxidizer, because it gains electrons. We know the byproduct of this reaction as rust. Rust is iron oxide—Fe(OH)2—and depending on factors like pH and salts, it comes in many forms.
We are not chemists, so this is complex stuff that we will simplify for our audience. But before we do, the following formula is one example of what it looks like when chlorine oxidizes iron:7
Iron oxide can stay in solution, but at some point, it can fall out of solution and stain. Usually this occurs at a higher pH and when the water is oversaturated. Naturally, that stain will be rust-colored. If you have rust stains in your pool, it’s probably iron oxide. It could also be iron bacteria.8
Sequestering vs. Chelating
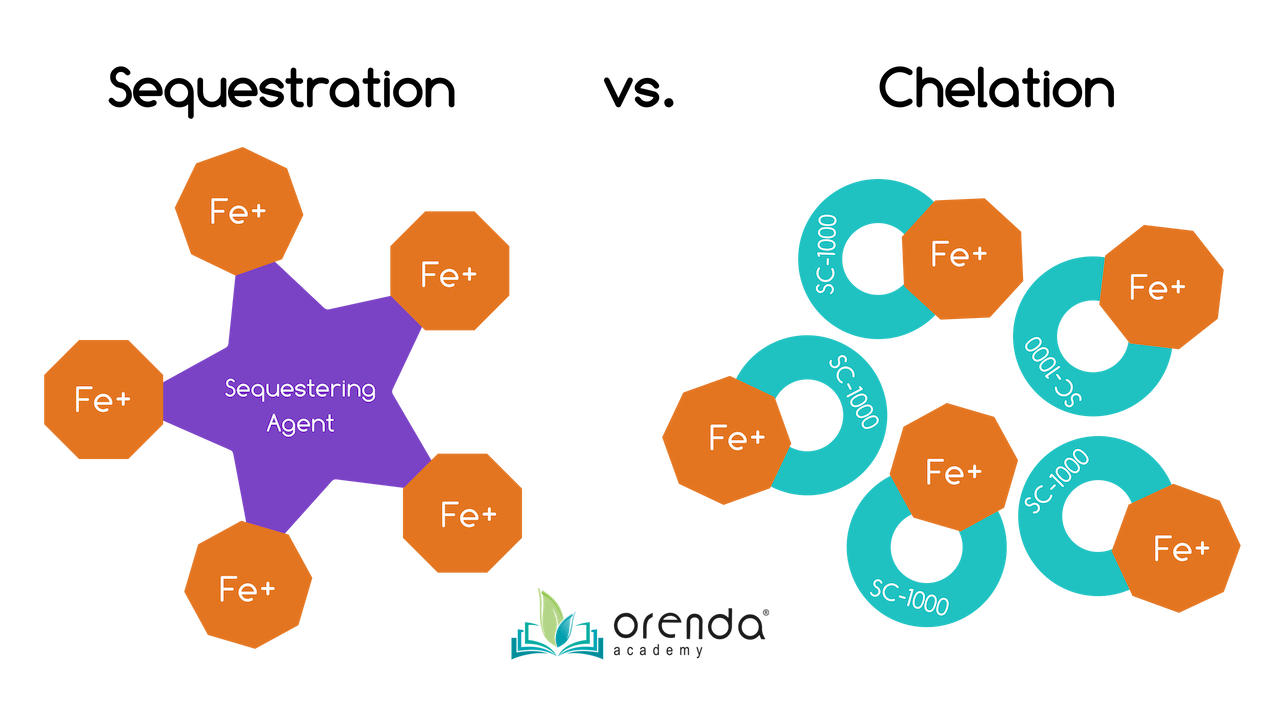
What is the difference between chelation and sequestration? Most metal control products in the pool industry are sequestering agents, and most of those sequestering agents are phosphate-based (phosphonic or phosphoric acid). Our metal control product, SC-1000, is a chelating agent that contains no phosphates at all.
Sequestering is like using a magnet. A sequestrant attracts heavy metals (like iron and copper) and minerals (like calcium). When they bond with the sequestering agent, these metals cannot be oxidized, and calcium cannot bond to carbonate ions to form calcium carbonate scale or dust. The metals and minerals are held in suspension (not solution), and sometimes the clusters are large enough to be filtered out. These amazing benefits are why phosphate-based sequestering agents are added to drinking water...they help control scale and metal issues, which protects the infrastructure of the treatment plant and the pipes.9
Chelation divides and conquers. Rather than attracting a whole bunch of metals and clumping them together, chelating agents divide up and attract individual ions of metals and minerals. Chelation occurs ion by ion, and holds metals and minerals in solution (not suspension). This is preferred for minerals like calcium, but is a disadvantage for trying to filter out and remove metals. Chelated metals tend to pass through filters because they are not sequestered together. Chelated metals are smaller in size than sequestered metals.
Is sequestering or chelating right for my pool?
If you are trying to capture and remove metals, then sequestering is a better solution. Used in conjunction with a metal filter, sequestering is a faster mechanism for grouping metals together into larger particles. However, sequestering agents are temporary because they can be oxidized over time, and break down in sunlight. And if the sequest is phosphate-based (like most of them are), PR-10,000 will wipe them out. So they are good for short-term remediation, but not necessarily a long-term solution.
Chelation is not as good for removing metals, but is excellent for keeping them in solution long-term. SC-1000 is immune to sunlight and chlorine degradation and therefore has excellent longevity in the water. It also contains no phosphates, so it is compatible with phosphate removers like PR-10,000. Chelation is a better long-term method of managing metals and preventing them from being oxidized. It is also preferred for managing minerals like calcium, which we want to stay in solution for LSI balance reasons.
Depending on your pool's circumstances, both sequestering agents and chelating agents have their place.
| Issue / Purpose | Solution | Best products |
| Heavy metals in the water | Capture and remove the metals | Tap water pre-filter, sequestering agent, metal-capturing filter |
| Metal stain | Address the source of the metals, identify the stain, lift the stain, bind the metals, and remove them | Citric/ascorbic acid, sequestering agent, metal-capturing filter |
| Stain prevention | Bind to and/or remove metals |
SC-1000 or sequestering agent, metal-capturing filter |
| Calcium carbonate scale | Balance LSI and keep calcium in solution |
SC-1000 |
| Discolored water | Diagnose with the white bucket test, then treat accordingly |
If metals, sequestering agent or SC-1000, metal-capturing filter |
How to prevent metal stains in a pool
Minimalist, preventative, proactive pool care is what we teach every day. We advocate for testing fill water and filtering metals out of the tap water that fills a pool. Also, we encourage plaster crews to filter out metals from the water they use to mix the plaster itself, lest those metals continue to oxidize within the pool’s surface and cause chronic staining problems.
To prevent metal stains (rust stains, for example), it’s all about keeping metal levels in the water low, and managing whatever metals you may have. This can be difficult when the tap water has high metal content in it. It’s even more difficult when using metal-based products. So we'll ask you: what can you do to minimize metals in your water?
For the metals that still get into your water, chelation or sequestration are both very effective. Chelation is better for long-term maintenance, whereas sequestration is better for short-term fixes. Our SC-1000 is excellent at stain prevention, but not as good as a sequestering agent at stain removal.
How to remove metal stains in a pool
Removing existing stains is a different issue, but similar steps to remedy.
- If you have stains, you should add SC-1000 or sequestering agent to the water. This will grab metals released during step 2.
- The second step is to try and lift the stain from the affected areas. You can accelerate the stain removal with ascorbic acid. Monitor the pH of the water when doing this step. Pool operators may want to vacuum or brush the stained area too. Try to apply the ascorbic acid (also called Vitamin C, or citric acid) evenly across the stain. If it's a small stain, use a PVC pipe that reaches the stain so that the granular acid lands on the stain only.
- Once the acid has had a chance to lift the stain, brush thoroughly.
Pool stain removal can take minutes or it can take weeks. There are too many variables that impact the process to discuss here. Moving forward from stain removal, follow the steps above for prevention, which should help eliminate the problem long term.
In full disclosure, stain removal is not Orenda's specialty. We are far better at preventing them than removing them. If you already have stubborn stains, consider more specialized products and techniques.
Conclusion
Pool stains are a nuisance, but an understanding of why they occur can help you manage them. Oxidation of these metals is inevitable in the presence of an oxidizer like chlorine. Rather than trying to prevent oxidation from occurring, we say minimize the amount of metals to begin with, and chelate what is left over.
1 Orenda products, by design, are proactive. Cleaning up stains after they occur is reactive, and our SC-1000 is not made for that. We'll explain later in this article what chelation means, but just know that we are not metal stain experts. There are a few other companies in the pool industry that specialize in metals and stains, and we encourage you to look at their information to learn more. This article is just an overview.
2 Rehman, K., Fatima, F., Waheed, I., & Akash, M. S. H. (2018). Prevalence of exposure of heavy metals and their impact on health consequences. Journal of cellular biochemistry, 119(1), 157–184. https://doi.org/10.1002/jcb.26234
3 Järup L. (2003). Hazards of Heavy Metal Contamination. British Medical Bulletin, 68, 167–182. https://doi.org/10.1093/bmb/ldg032
