Does chlorine affect pH?

Chlorine products have different pH values when dissolved in water, but their impact on the pH of a swimming pool is not always significant. This article discusses how each chlorine type impacts pH and why.
Covered in this article:
- Different types of chlorine products and their pH impacts
- Liquid chlorine (sodium hypochlorite)
- Cal hypo (calcium hypochlorite)
- Why does cal hypo cloud the water?
- Dichlor (sodium dichloro-s-triazinetrione dihydrate)
- Trichlor (trichloro-s-triazinetrione)
- Saltwater Chlorine Generators (SWG)
- Chlorine gas (Cl2)
- Chlorine's formation of Hydrochloric Acid (HCl)
- Containing pH in a chlorine pool
- Conclusion
Different types of chlorine products and their pH impacts
Chlorine products each affect water chemistry differently. So let's quickly review the most popular types of chlorine products used in swimming pools.
- Liquid chlorine (sodium hypochlorite)
- Cal Hypo (calcium hypochlorite)
- Trichlor (trichloro-s-triazinetrione)
- Dichlor (sodium dichloro-s-triazinetrione dihydrate)
- Salt chlorine generators
- Chlorine gas (Cl2⇡)
Related: Pool Sanitizers, part 1: Chlorine
1. Liquid chlorine (sodium hypochlorite)
.png?width=760&height=428&name=4.9%20Water%20Chemistry%20-%20Total%20Dissolved%20Solids%20(TDS).png)
Liquid chlorine is manufactured by dissolving chlorine gas in sodium hydroxide (caustic soda). The higher the pH of the solution, the higher the percentage of Hypochlorite ion (OCl-) compared to Hypochlorous Acid (HOCl). Hypochlorite is far more stable and decomposes slower than HOCl, so excess sodium hydroxide and water are added to keep the pH around 12-13. This higher pH extends the degradation curve of the product, extending its shelf life.1
The sodium hypochlorite chemical manufacturing process looks like this:
Cl2⇡ + 2NaOH → NaOCl + NaCl + H2O
Chlorine (gas⇡) + 2 Sodium Hydroxides → Sodium Hypochlorite + Salt + Water
Due to this excess sodium hydroxide, liquid chlorine will temporarily raise the pH of a pool. The pH rise is only temporary because when the killing form of chlorine (HOCl) oxidizes, kills, or breaks down in sunlight, it becomes hydrochloric acid (HCl). This hydrochloric acid brings the pH back down, neutralizing the temporary high pH. We will discuss this process later in this article.
When liquid chlorine is added to water, the following reactions occur:
NaOCl + H2O → HOCl + NaOH
Sodium Hypochlorite + Water → Hypochlorous Acid + Sodium Hydroxide
Shortly afterward, the sodium hydroxide separates, so another way to show this same reaction is:
NaOCl + H2O → HOCl + Na+ + OH-
Sodium Hypochlorite + Water → Hypochlorous Acid + Sodium ion + Hydroxide ion
As you can see, the active form of chlorine, Hypochlorous acid (HOCl) is formed, and the high-pH sodium hydroxide (NaOH) is a byproduct. Keep this in mind later in this article when we explain what chlorine does after it dissolves. Over time, liquid chlorine's pH impact is almost neutral.
2. Cal Hypo (calcium hypochlorite)

Calcium hypochlorite (Ca(OCl)2) dissolves in water to create HOCl and a high-pH byproduct called calcium hydroxide (Ca(OH)2). When dissolved in water, it has a pH of 10-11.8, depending on the concentration of the product. Like liquid chlorine, cal hypo creates a temporary rise in pH.
Cal hypo can be produced in a couple of ways. One of them is by reacting chlorine gas with calcium hydroxide.2 Here's the chemical reaction:
2Cl2⇡+ Ca(OH)2 → Ca(OCl)2 + 2HCl
2 Chlorines (gas⇡) + Calcium hydroxide → Calcium hypochlorite + 2 Hydrochloric acids
When cal hypo is added to water, the following reaction occurs:
Ca(OCl)2 + 2H2O → 2HOCl + Ca(OH)2
Calcium hypochlorite + water → 2 Hypochlorous acids + Calcium hydroxide
As you might imagine, cal hypo's long-term pH impact in water is virtually identical to liquid chlorine: almost neutral. Yes, it temporarily increases pH (and therefore, it also increases LSI where you pour it in). Still, once chlorine is used or broken down in sunlight, the released HCl almost completely neutralizes the high pH. That's why cal hypo is virtually pH-neutral over time.
Why does Cal Hypo cloud the water?
Cal hypo clouds in the water because it forces a high-LSI violation locally (where you are pouring it in). In the short term, it spikes the pH, converting bicarbonate ions (HCO3-) into carbonate ions (CO32-). Carbonate ions bind to calcium ions (Ca2+) to create calcium carbonate (CaCO3). Boom, cloudy water. This is similar to why soda ash clouds water.
Related: Why does soda ash cloud a swimming pool?
Furthermore, cal hypo introduces a high concentration of calcium, too. So not only is it a high pH, it's also a high concentration of calcium. Both of which increase the LSI dramatically. To reduce cal hypo clouding, try these steps:
- reduce pH to below 7.8 with diluted acid a few minutes before adding cal hypo
- rinse out the bucket thoroughly after using acid. (Do not shortcut this step!)
- pre-dissolve cal hypo in the bucket and dilute it
- pour in the dissolved cal hypo around the pool's perimeter, slowly.
- If it clouds, slow down and dip the bucket into the pool to let water into the bucket to further dilute it. Then, move to another section of the pool to continue adding it.
3. Dichlor (sodium dichloro-s-triazinetrione dihydrate)
When dissolved in water, sodium dichlor dihydrate has a fairly neutral pH between 6.0 and 7.0, depending on the purity of the product.3 While it's close to perfectly neutral (7.0 pH), dichlor's pH is slightly lower than the desired pH levels of a swimming pool. Therefore, it will slightly reduce pH of the pool. Dichlor will not have a noticeable impact on total alkalinity.
As discussed in our previous article, the main byproduct left behind by sodium dichlor is cyanuric acid. By weight, only 27.7% of dichlor is chlorine, while 49.2% is cyanuric acid.4 One pound of dichlor in 10,000 gallons of water increases the CYA by about 6-6.5 ppm, depending on the purity of the dichlor product.
4. Trichlor (trichloro-s-triazinetrione)

So far, all three chlorine types we have covered have almost no long-term impact on pH. Yes, liquid chlorine and cal hypo temporarily increase pH, but the HCl produced will neutralize that high pH. Sodium dichlor dihydrate will temporarily decrease pH, but the pH will naturally rise thanks to Henry's Law and the loss of carbon dioxide.
Trichlor, however, is different. Trichlor lowers the pH and alkalinity of the pool.
Trichlor comes in either slow-dissolve (erosion) tablets or sticks, or as a granular shock. The erosion tablets (commonly referred to as "tabs") break down slowly, releasing the product over several days or possibly weeks. The granular shock dissolves pretty quickly.
Regardless of the trichlor product used, the pH is strongly acidic, between 2.8 and 3.0. Because of this, trichlor will lower the pH and total alkalinity in a swimming pool.
When trichlor dissolves, its acidity neutralizes bicarbonate alkalinity––meaning it adds a Hydrogen ion (H+) to bicarbonate ion (HCO3-), converting it into carbonic acid (H2CO3).5 This conversion of bicarbonate ions into carbonic acid reduces the Total Alkalinity, and the subsequent increase in carbonic acid lowers the pH. Carbonic acid is dissolved carbon dioxide (H2O + CO2 → H2CO3).
Related: What is Carbonate Alkalinity?
Additionally, like dichlor, Trichlor's main byproduct is cyanuric acid. By weight, only 45.8% of trichlor is chlorine, while over 53% is cyanuric acid (this depends on the purity of the product, as there may be some binding agents and fillers). One pound of trichlor in 10,000 gallons of water increases the CYA by about 6-6.7 ppm, depending on the purity of the trichlor product.6
5. Saltwater chlorine generators (SWG)
Anyone who has managed a saltwater pool knows that the pH rises. The perception is the pH rises indefinitely, but in reality, the pH of a saltwater pool can only rise to the pH ceiling.
The common belief is that salt chlorine generators raise pH chemically because of the high-pH byproduct (sodium hydroxide, NaOH) produced. And while NaOH is produced, that's not what actually raises the pH in the pool. The truth is that saltwater-generated chlorine is the most pH-neutral chlorine you can use in a pool.
We explain saltwater systems in depth in our other article here. Below is an illustration explaining why some salt systems produce calcium flakes. Focus on the first illustration (top left), to see the chemistry of a salt cell.
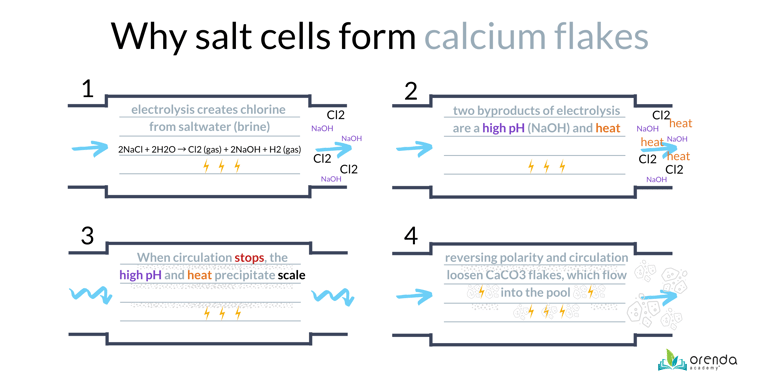
Salt dissolves in water, separating NaCl into sodium and chloride ions (Na+ and Cl-). When saltwater flows through an active salt cell, electrolysis attracts the sodium to the negative side (cathode), and the chloride to the positive side (anode).7 The cathode produces Hydrogen gas (H2⇡) and sodium hydroxide (NaOH). The anode produces Chlorine gas (Cl2). It goes like this:
Cathode (-)
2H2O + 2(e-) → H2⇡ + 2OH-
2 Waters + 2 electrons → Hydrogen (gas⇡) + 2 Hydroxides
Simultaneously, the sodium ion loses its charge:
Cathode (-)
Na+ + (e-) → Na
Sodium ion + electron → Elemental sodium
The anode, which attracted the Chloride ion (Cl-), creates chlorine gas (Cl2), as follows:
Anode (+)
2Cl- → Cl2⇡ + 2(e-)
2 Chloride ions → Chlorine (gas⇡) + 2 electrons
Now let's combine these reactions so we can show exactly what salt systems convert saltwater into:
2NaCl + 2H2O → 2NaOH + H2⇡+ Cl2⇡
2 Salts + 2 Waters → 2 Sodium hydroxide + Hydrogen (gas⇡) + Chlorine (gas⇡)
Salt-generated chlorine is pH neutral because sodium hydroxide's high pH neutralizes chlorine gas's low pH down the line from the salt cell. The pH rises in saltwater pools because of physics, not chemistry. The hydrogen gas bubbles create turbulence, promoting carbon dioxide loss, which raises the pool's pH.
6. Chlorine gas (Cl2⇡)
Let's wrap up our summary of chlorine types with chlorine gas. It should be noted that chlorine gas is rarely (if ever) used in swimming pools anymore because it has been outlawed in most places. We are including it because chlorine gas is strongly acidic when dissolved in water. Here's why:
Cl2⇡ + H2O → HOCl + HCl
Chlorine (gas⇡) + water → Hypochlorous acid + Hydrochloric acid
Technically speaking, dry and gas chemicals do not have a pH, because pH is based on water's interaction with these substances. So when chlorine gas dissolves, it creates two acids: active chlorine, hypochlorous acid (a weak acid), and hydrochloric acid (a strong acid).
If you were to add chlorine gas to a pool, it would lower the pH and total alkalinity.
Chlorine's formation of Hydrochloric Acid (HCl)
As mentioned in several previous sections of this article, every chlorine product will dissolve in water and produce Hypochlorous acid (HOCl) and some byproducts. That HOCl will be used or broken down in sunlight to produce Hydrochloric acid (HCl). Here's a graphic from Bob Lowry illustrating the reactions:8
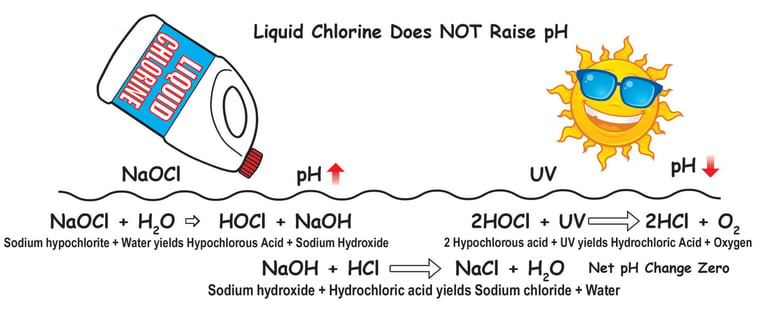
Credit: Robert Lowry, Pool Chemistry Training Institute
For high-pH chlorine types like sodium hypochlorite and calcium hypochlorite, this newly created HCl almost fully neutralizes the hydroxide byproducts left behind. That's why these chlorines are virtually pH-neutral over time. They only temporarily raise the pH.
Containing pH in a chlorine pool
So what can we do about this? Should we treat pools differently based on the primary chlorine type used?
Yes. But the differences are small. If you are familiar with our Orenda Program, you know we teach
- proactive pool care, with
- no chemical conflicts, and
- no harmful long-term byproducts left behind.
We teach our customers to focus on putting water in its natural state, but doing so on our own terms. If we disregard water's need for LSI balance, water will balance itself...and it will do so in a way that makes disinfection and overall water quality more difficult. We describe this concept in detail in episode 132 of our podcast:
All that is to say, we can standardize our pools in a given area based on a few factors. In our program:
- The water temperature extremes determine your ideal calcium hardness level throughout the year. This is closely correlated with where an outdoor pool is located, and/or the temperature setpoint for an indoor pool.
- Your ideal total alkalinity level is determined by the primary chlorine type used. Saltwater, liquid chlorine, and cal hypo pools should have TA levels around 60 ppm, usually not above 70 ppm. But do NOT do this without sufficient calcium hardness to offset this reduced TA and maintain LSI balance.
- The total alkalinity level will drive the carbonate alkalinity level, which determines the pH ceiling of your water.
- Unless using trichlor as a primary chlorine––which we strongly recommend against9––the pH will naturally rise due to the loss of CO2. Predict it and act accordingly.
If the fundamentals of LSI water balance are in place, we can use physics to contain the pool's pH.
Conclusion
Except for trichlor, swimming pool chlorine products do not noticeably impact pH over time. Yes, the hypochlorites temporarily increase pH, but they also release Hydrochloric acid (HCl) to neutralize the hydroxide byproducts.
Saltwater chlorine generators are chemically pH neutral, but thanks to the turbulence created by hydrogen gas (H2⇡) bubbles, Carbon dioxide (CO2⇡) will be released. This causes the pH to rise to the pH ceiling thanks to physics.
Hopefully, this article has shed light on what happens when chlorine is added to a swimming pool.
1 OxyChem (2014). Sodium Hypochlorite Handbook.
2 Ropp, R.C. (2013). Group 17 (H, F, Cl, Br, I) Alkaline Earth Compounds - Calcium Hypochlorite. Encyclopedia of the Alkaline Earth Compounds. Chapter 2, (pp. 25-104). https://doi.org/10.1016/B978-0-444-59550-8.00002-8
3 This slideshow from the US EPA indicates the pH of both sodium dichlor anhydrous and sodium dichlor dihydrate as 6.0 to 7.0, in a 1% solution. Because of its volatility, we don't use dichlor anhydrous in the pool industry. All pool dichlor products are sodium dichloro-s-triazinetrione dihydrate. The EPA presentation is cited here, with a downloadable PDF:
Wahman, D. (2018). Dichlor & Trichlor Water Chemistry Implications. US Environmental Protection Agency, 15th annual EPA Small Systems Workshop. Slide 10. Downloadable PDF here.
4 We show the math for this (and all other chlorine products) in our previous article, here.
5 Wojtowicz, J. (2001). The Carbonate System in Swimming Pool Water. Journal of the Swimming Pool and Spa Industry. Vol. 4(1), pp. 54-59.
6 We display precise chlorine byproducts in the Orenda Calculator™ dosing results. The math assumes the product is 99% pure trichlor like it is labeled. Assuming the product is as pure as its label indicates, one pound of trichlor increases the chlorine by 11 ppm in 10,000 gallons; it increases CYA by 6.68 ppm.
7 Electrolytic Cells. Bodner Research Web, Purdue University Chemistry Department.
8 Lowry, Robert W. (2021). Why pH rises if not from liquid chlorine? Pool Chemistry Training Institute - Technical Bulletin.
9 Trichlor was originally designed to be a supplemental chlorine product. The three main issues with using trichlor as a primary chlorine type are CYA overstabilization, constantly decreasing TA, and a suppressed pH.
