Ideal Calcium Hardness and Total Alkalinity Levels in Pools

What should your calcium hardness be? What should your total alkalinity be? Are there ideal numbers? And if so, what are they for your pool? This article discusses these questions through the lens of overall water balance.
Covered in this article:
- Range chemistry vs. the LSI
- What is the ideal calcium hardness level?
- What is the ideal total alkalinity level?
- Conclusion
First, for context, let's review the differences between range chemistry and the Langelier Saturation Index (LSI).
Range chemistry vs. the LSI
Traditional pool chemistry is taught using ranges for each parameter, (e.g. pH should ideally be 7.4-7.6, total alkalinity 80-120 ppm, calcium hardness 200-400 ppm, etc.). We at Orenda call this range chemistry, which we argue matters less than LSI balance. Individual parameters are fine, as long as they are used within the context of the LSI as a whole.
To maintain LSI balance year-round, textbook ranges do not always work. Here's a screenshot showing how 'perfectly balanced' water (based on textbook ranges) fails to maintain LSI balance in cold water:
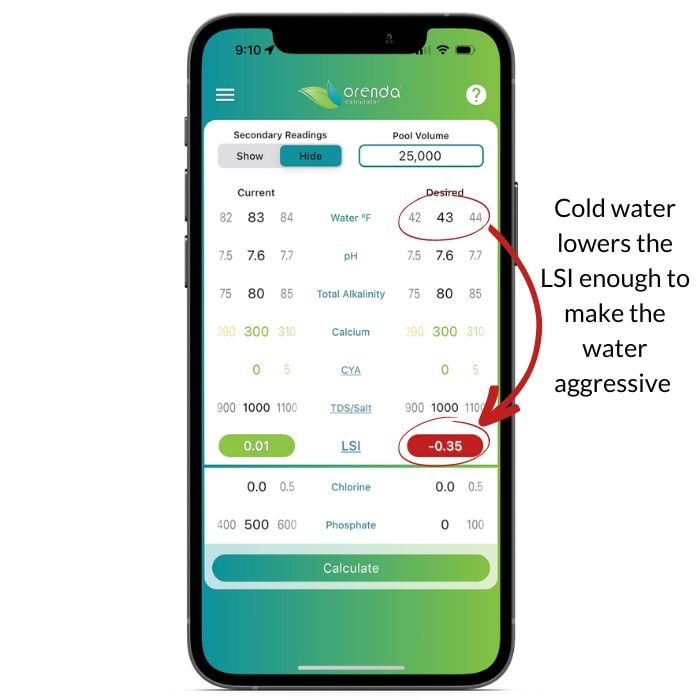 Now we have context for the main topic for this article. We are about to introduce a concept to you that you may not have ever heard of unless you have taken one of our classes at a trade show or private training event. The principal concept is to contain pH while maintaining LSI balance. The two can be difficult to do at the same time unless you have a favorable ratio of calcium hardness to total alkalinity (CH: TA). Let's dive in.
Now we have context for the main topic for this article. We are about to introduce a concept to you that you may not have ever heard of unless you have taken one of our classes at a trade show or private training event. The principal concept is to contain pH while maintaining LSI balance. The two can be difficult to do at the same time unless you have a favorable ratio of calcium hardness to total alkalinity (CH: TA). Let's dive in.
The calcium to alkalinity ratio (CH:TA)
We first learned this concept from Tom Carrico, and it has stuck with us ever since. Tom told us that a man named Dr. McNamara used to advise the NY state health department many years ago, and he was a water chemistry expert. Dr. McNamara reportedly discovered an optimal ratio of calcium hardness (CH) and total alkalinity (TA) for pool management; specifically for injecting carbon dioxide for pH control. That ideal CH: TA ratio is 4:1. Whether Dr. McNamara originated the idea or got it from someone else, we may never know.1
As mentioned in our footnote, we would cite our sources here, but we cannot find anything published by Dr. McNamara, or even mentioning him by name. So what we're sharing here is our opinion, and it comes from our own field experience. For the scientists out there, let's call it empirical evidence.
When Tom told us about the 4:1 ratio, rather than doing our research, we simply listened and applied this ratio wherever we go. It has been undeniably successful in the field. We have found that pH is more predictable and fluctuates less, LSI balance is more easily maintained, and chemical consumption–specifically acid and sodium bicarb–is noticeably reduced when compared to traditional pool chemistry.2
We have found a 4:1 (or higher) CH: TA ratio consistently improves our customers' ability to control their water IF the values balance the LSI. That deserves repeating. The ratio only works if the values balance the LSI. If not, you're defeating the purpose and the chemistry will be out of balance anyway.
For instance, you could have a 4:1 ratio of 100 ppm CH and 25 ppm TA, but your LSI would be very low and the water would be extremely aggressive. Conversely, you could have 800 ppm CH and 200 ppm TA and you'd have a very high LSI leading to scale formation.
Keep in mind that this 4:1 ratio must be within the context of the LSI. If so, it renders 'chasing pH' obsolete, because with the right CH: TA ratio, you can contain pH instead of trying to control it.
We should add that the 4:1 ideal ratio is not always necessary for successfully maintaining your pool. We at Orenda have found that 3:1 calcium to alkalinity is a viable minimum ratio. We have also found that going above 4:1 (e.g. 4.5:1, 5:1, 7:1, etc.) also works phenomenally well, especially in colder temperatures. Refer back to our LSI target ranges chart above and you can see our TA and CH numbers are broad enough to encompass these ratios.
But why? Why are we observing such success with these ratios?
To find out, we did some digging, and we talked to chemists and others who know way more than we do about water. Our first expert–Richard Falk–in no uncertain terms obliterated the validity of the CH: TA ratio. It was actually so much of a stomp down it was funny to read...
The CH: TA ratio itself is a myth...but it helps frame our chemistry strategy
You might find it odd to publish a concept that has no chemistry validity whatsoever, but we think it's fantastic. Here's what you need to know: this ratio itself is not why pool chemistry gets easier. The ratio is a just teaching tool that helps us develop a good chemistry strategy. Specifically, we need more calcium hardness to offset lower total alkalinity.
So while we still do not know what Dr. McNamara was talking about with his 4:1 ratio, we do know that we have seen lower TA and higher levels of CH work very well for pool chemistry. Think of the ratio as a helpful reminder to have enough calcium hardness in your water to maintain LSI balance at any given temperature.
Related: Total Alkalinity vs. pH
The ratio alone is not enough to bring you success in the field. As mentioned before, you could have a 4:1 ratio that is absurdly high or absurdly low on the LSI, and that defeats the purpose. What you really need is an LSI balance with lower carbonate alkalinity to reduce your pH ceiling. See the chart below to remind you of the pH ceiling:
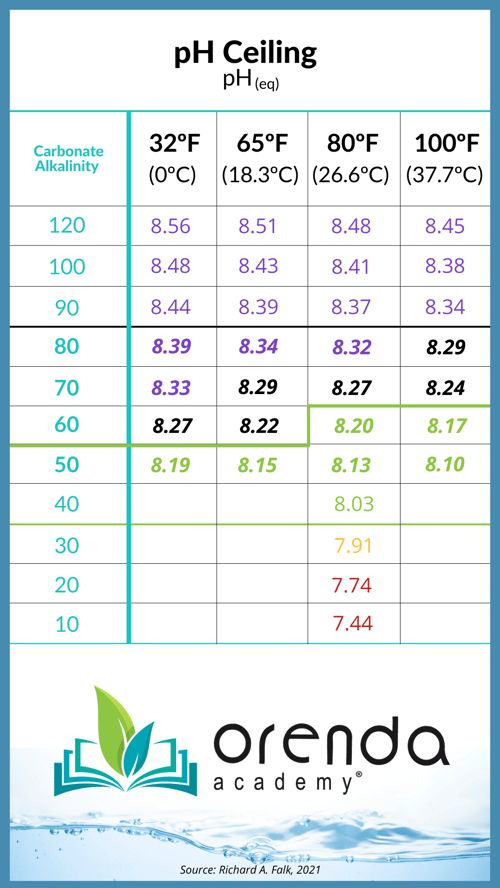 The lower your carbonate (corrected) alkalinity, the lower your pH ceiling, and therefore the easier it is to contain pH. But in order to have lower carbonate alkalinity, you need more calcium hardness to compensate for it. From Richard Falk:
The lower your carbonate (corrected) alkalinity, the lower your pH ceiling, and therefore the easier it is to contain pH. But in order to have lower carbonate alkalinity, you need more calcium hardness to compensate for it. From Richard Falk:
"...the better way to look at it is not as a ratio, but to lower the carbonate alkalinity. And when one does that, raise the calcium hardness to compensate." - Richard Falk3
What seemed like a chemistry phenomenon turns out to be pure coincidence. So to reiterate one final time, we're saying to use the 4:1 (or higher) ratio as a rule of thumb target to help you simplify your water chemistry, even though there's only an indirect connection between calcium hardness and total alkalinity. Here, we explain it better in this Rule Your Pool podcast episode:
What is the ideal calcium hardness level for a swimming pool?
With the ratio concept in mind, the ideal calcium hardness level is whatever allows you to maintain LSI balance year-round in your pool. In warmer climates, the number will be lower. In colder climates, it will be higher.
What is the ideal total alkalinity level for a swimming pool?
The primary sanitizer type will drive the desired total alkalinity level. Salt pools, for example, should have less total alkalinity than a trichlor pool. Once you have found your water temperature and primary chlorine type, play around with the Orenda App's LSI calculator to find specifically what works for you and your pool. The ideal total alkalinity level is enough to buffer against acid, but not high enough to drive the pH over 8.2. For that, refer to the pH ceiling chart above and figure out your carbonate alkalinity.
Conclusion
Use the calcium hardness to total alkalinity ratio (CH: TA) as a reminder to have a lower TA in certain pools, but simultaneously have higher calcium hardness to offset it. If you do, containing pH will be easier (because the pH ceiling will be lower). Maintaining LSI will also be easier because you have a strong foundation of calcium hardness in your water, and it won't change easily. It works phenomenally well.
1 At this point, we are smiling about this. It reminds us of how knowledge and wisdom were passed down through generations prior to recorded history being written. We never knew Dr. McNamara, and as far as our online searches have gone, we cannot even prove he existed. But we heard about him from one of our commercial dealers–a very credible source–who relayed the information he learned first-hand many years ago. Because of the lack of published documentation, what we are sharing today is based on our own experience in the field, trying different things with various customers, and tracking the results. The results speak for themselves, and this concept has proven to be true and effective. So here's to you, Dr. McNamara. Thank you for sharing this idea with others, because now we know about it and can share it with the world. We pride ourselves on vetting information and citing/crediting our sources. But in this case, we thank Tom Carrico for sharing it with us, and we credit Dr. McNamara for the concept.
2 See our 6 bad habits article. Bad habit #2 is chasing pH. When we say "traditional pool chemistry" in this article, we are referring to the normal chasing of pH each week. Such chasing is futile and uses more acid and sodium bicarbonate than necessary. Contrast this pH chasing with the Orenda program of embracing physics, containing pH, and utilizing good calcium to alkalinity ratio, and the difference is obvious.
3 Quoted from an email conversation with Richard Falk. And just for fun, here's more of what Falk said: "As I noted above, the ratio concept is COMPLETELY bogus since one can readily achieve 4:1 with 200 ppm TA and 800 ppm CH and yet that's obviously nuts since it has too high a calcite saturation index AND has high carbon dioxide outgassing with pH rising quickly due to the high TA. Again, the ratio idea is completely insane." Richard knows how to cut through the crap and tell it like it is. And we love it.
