Why Swimming Pool Temperature Matters

Swimming pool temperature impacts the water chemistry in a big way. This article will outline a few reasons why.
Covered in this article:
- Pool temperature and water quality
- Oxidant Demand
- Pool Temperature and Water Balance
- Water temperature is the LSI's most ignored factor
- Temperature is the moving baseline for an LSI strategy
- Cold water issues
- Warm water issues
- Conclusion
Pool temperature and water quality
Let's divide water chemistry into two different categories: water quality and water balance. Let's focus on water quality for now.

Warmer water means more chlorine demand. This is not due to sunlight degradation of chlorine, though summertime does mean more hours of direct sunlight hitting an outdoor pool. The real reasons for higher chlorine demand are that both living and non-living contaminants are more prevalent in warmer water.
Algae and bacteria, for instance, are living contaminants that chlorine must kill (sanitization). Warmer water means those microorganisms can reproduce more rapidly. Therefore, problems like algae are more prevalent in the summertime. If you have a pool, you probably already know this.
To kill any living organism, chlorine needs a certain amount of Contact Time (CT). CT is influenced by pH (in a non-stabilized pool) and water temperature. Why? Because warmer temperatures increase chlorine performance. Here's a quote from the water research center:
"To determine CT(required) we need to know:
1) The minimum temperature of the water during disinfection. The minimum temperature of the water in the chlorine contact chamber must be monitored. Minimum temperature is used because chlorine’s ability to disinfect becomes less with lower temperatures. By using the lowest temperature of the water when determining CT(required) we know that the disinfection that occurred was at least as good as the lowest temperature allowed.
Non-living organics, which are the bulk of the pool's oxidant demand, are more prevalent in warm water. Warmer weather means more swimmers and more non-living organics like sunscreen and body oils. Between these organics and the living contaminants like algae, warm water has a higher chlorine demand than cold water.
Chemical reactions generally work faster at higher temperatures. Water chemistry is no different. So chlorine will work better and faster, and as a result, chlorine will also get reduced faster.
Oxidant Demand
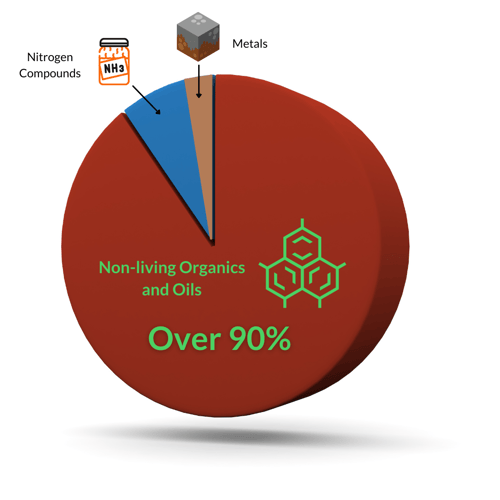
There are three categories: metals, nitrogen compounds, and non-living organics. Of these, non-living organics and oils dominate the list of oxidants in swimming pools.
Metals are the easiest for chlorine to oxidize, so they are the first to go. Nitrogen compounds like ammonia and urea are much more difficult to oxidize. They require a breakpoint chlorination process where chlorine combines with these compounds before destroying them. Non-living organic compounds are various hydrocarbons that can be complex.1
Water temperature impacts the speed of chemical reactions. The colder the water, the slower the reactions. In the case of chlorine, cold water slows oxidation rates and therefore lowers the ORP. Temperature directly affects all chemical reactions in water, especially chlorine. And unlike living contaminants like germs and algae, oxidants do not reproduce themselves. So, while the sanitizer demand slows down in cold water, the oxidant demand does not.
Pool temperature and water balance
Water balance is all about physics. It's measured using the Langelier Saturation Index (LSI), which tells us the water's saturation equilibrium of calcium carbonate (CaCO3). Water temperature plays an important role in LSI balance. The colder the water, the lower the LSI. In other words, colder water will be more aggressive. Cold water needs more calcium than warm water.
Related: LSI Balance and Calcium Management (PIllar 1)
Water temperature is the most ignored LSI factor
Of the six LSI factors, water temperature is the easiest and most reliable factor to measure. All it takes is a thermometer. Most people––pool professionals and homeowners alike––are unaware that water temperature makes as big of a difference as it does.2 And yet, temperature is the most ignored LSI factor.
We were unaware of water temperature's importance until we built the Orenda Calculator™ and saw it for ourselves. Take a look:
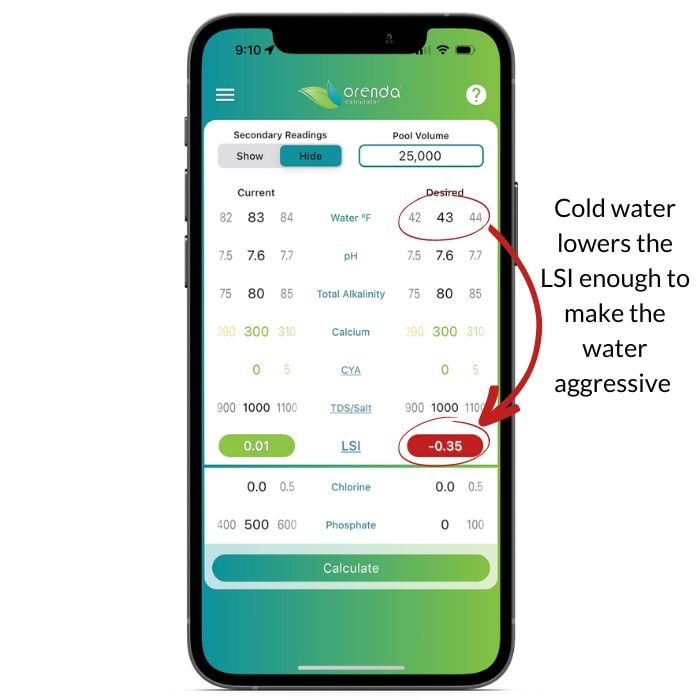 In this scenario, a 35º drop in pool temperature changes the LSI from balanced to aggressive. It was perfectly balanced at 80ºF, but at 45ºF, the water will etch a surface (and fade a vinyl liner, degrade a fiberglass gelcoat, etc.).
In this scenario, a 35º drop in pool temperature changes the LSI from balanced to aggressive. It was perfectly balanced at 80ºF, but at 45ºF, the water will etch a surface (and fade a vinyl liner, degrade a fiberglass gelcoat, etc.).
Fortunately, the water temperature does not drop down so dramatically overnight. It takes weeks, if not months, for a swimming pool to drop 35º. This gives pool owners and operators time to prepare and predict how to handle it. And that brings us to our next point...
Water temperature is the moving baseline for an LSI strategy
We can predict that water temperature will drop in the autumn and rise in the spring. Use the pool's temperature as the baseline for every chemical treatment. This baseline moves, albeit slowly, allowing water chemistry to be adjusted throughout the seasons.
Most LSI factors can be adjusted with chemicals, but pool temperature, apart from cranking up the pool heater, is something you cannot easily adjust. Especially in the off-season. So, we at Orenda strongly suggest predicting the worst-case scenario for your off-season. If you're in a climate where the pool is guaranteed to freeze, that means setting your LSI parameters to be balanced at 32ºF (0ºC), so when the temperature drops that low, you have enough calcium and alkalinity to keep the water happy. If you don't, there will be consequences...
Cold water issues
We have other articles about cold water and the LSI. Most pool surface damage occurs during the winter.

The most common winter issues we see in plaster pools are calcium-related. Most people see calcium deposits and immediately think, "SCALE!!" But winter damage is hardly ever scale. These calcium deposits are usually calcite crystals or winter dust. These issues occur because of a low-LSI violation, and water had to seek equilibrium by dissolving calcium from the cement pool surface. When it did, the pH of the water spikes high.3 The water finds equilibrium at the coldest point and stops eating the walls.

In the case of calcite crystals, we believe the stagnant water provides a unique environment where calcification "grows" out of the cement surface as water eats away at it. The high pH and stagnant water seem to allow the calcium to re-calcify after it has been dissolved from the plaster surface. In the case of winter dust, the problem occurs in the spring as the water temperature begins to rise again. The pool will go from its coldest point to increasing by 30ºF or more, which makes a dramatic difference on the LSI. The water becomes over-saturated with calcium because the temperature is warmer now, so it deposits calcium in the form of winter dust. By the time the pool is opened, there could be winter dust everywhere.
In vinyl and fiberglass pools, LSI damage is arguably worse. Fiberglass pool gelcoats can be degraded and appear hazy, which we call "chalking". Vinyl liners lose pigment and fade, and they become more porous. Believe it or not, there is a small amount of calcium carbonate in vinyl, and hungry water wants it. A more porous vinyl liner is more prone to wrinkling as temperatures change, thanks to thermal expansion and contraction.
The bottom line: colder water means a lower LSI, which means water is more hungry for calcium. Either we feed the water what it needs, or the water will have to feed on your pool surface. This is why it's so important to winterize a pool properly, with the LSI in mind. If you are debating whether or not to have winter pool service, we recommend you do.
Orenda Procedure: How to Winterize a Swimming Pool
Warm water issues

On the opposite side of the temperature spectrum, we have scale-forming problems. The higher the temperature, the more likely calcium carbonate will fall out of solution as carbonate scale. This is why you first see scale in a warm spa, on the sunny tile line, or spillway. Calcium carbonate tends to precipitate in the hottest places first. The higher the temperature, the higher the LSI. So water temperature determines where carbonate scale will form first.
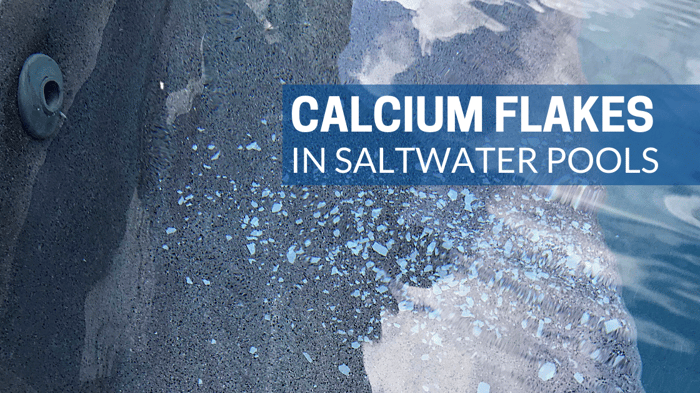
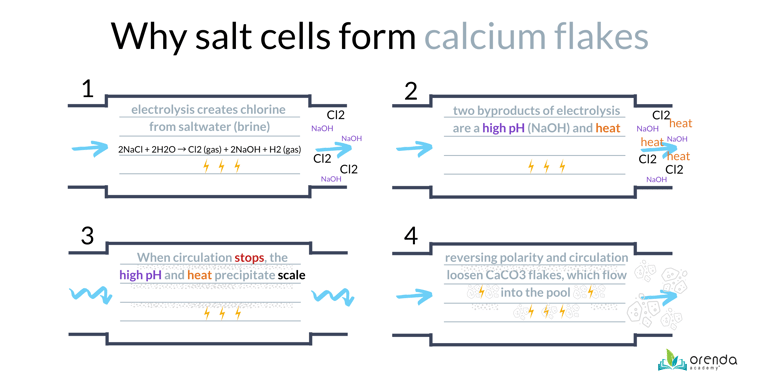
If you have a saltwater pool, your salt chlorine generator will scale before you see it in the pool itself. The consequences of this are what we call calcium flakes, or snowflaking (see photo). White chunks of calcium carbonate flake off the salt cell and blow into the pool through the return inlets. This is a very common LSI violation. Temperature is one of the factors that cause saltwater calcium flaking. Salt cells have very high pH inside, as well as heat caused by electrolysis.
Similarly, heat exchangers will scale too. Again, because of the high temperature. LSI violations occur locally, not everywhere in the pool system at once. So carbonate scale will likely precipitate wherever there is a purple LSI violation.4
Conclusion
Water temperature is the moving baseline for a good water chemistry strategy. For water quality, warmer water means faster chemical reactions and an increased sanitizer demand. For water balance, the temperature directly impacts the LSI balance of the water, and it needs to be accounted for. So use a thermometer and do not neglect temperature.
1 The complexity of non-living organics and oils is why we encourage the use of enzymes in water. Enzymes break down and remove carbon bonds, which noticeably improves water quality and clarity. Enzymes reduce chlorine demand directly because they lower the energy level required for chlorine to oxidize the material. It's easier for chlorine to oxidize the components of a complex hydrocarbon than the entire molecule itself. The most complex non-living organics are synthetic materials like deodorants, cosmetics, soap residue, lotions, and the king of chlorine demand, sunscreen.
2 Water temperature does not just impact the overall LSI balance of water. Water temperature matters for the accuracy of water testing, too. Cold water samples can distort reagents and give inaccurate test results.
3 The most soluble form of calcium in cement is calcium hydroxide (Ca(OH)2). It has a high pH of 12.6, so when low-LSI water dissolves it, the pH of the water increases strongly. It is common to have a pH in the pool above 10 when the LSI is in the red on the Orenda Calculator™ because the calcium hydroxide is spiking the pH up.
4 Purple LSI refers to the Orenda Calculator™, where numbers are color-coded. Purple means the LSI value is <+0.30. Green is from 0.00 to +0.30. Yellow is from -0.01 down to -0.30, and red is below -0.30.
