Why Orenda Advice Differs from Pool Industry Standards

Our water chemistry program defies swimming pool industry standards, textbooks, and pool stores. This article explains why we are different.
Covered in this article:
- Water balance vs. water quality
- Range Chemistry (water balance)
- What's based on range chemistry?
- Education and Textbooks
- Warranties
- Health codes
- Pool store testing software
- Conventional wisdom and habits
- Water balance, the Orenda way
- What's based on range chemistry?
- Overchlorination (water quality)
- Chlorine demand
- Sanitizer demand
- Oxidant demand
- Chlorine byproducts
- Water Quality, the Orenda way
- Chlorine demand
- Conclusion
Water Balance vs. Water Quality
There are two sides to water chemistry: water balance and water quality. We could talk forever about these two separate concepts, but this article will focus on where we differ from our industry's standards:
- Range chemistry (water balance)
- Over-chlorination (water quality)
Water Balance is what water cares about. We are different because unlike most of our industry, our teachings are not based on firm chemistry ranges, which we hereafter refer to as "range chemistry". In our opinion, the Langelier Saturation Index (LSI) is the best measurement of water balance. Most other water industries use the LSI, and we think the pool industry needs to also. And while Dr. Wilfred Langelier originally created the LSI for closed-water systems like boilers and cooling systems, it has since been adapted for swimming pools to account for things like Cyanuric Acid (CYA). We teach water balance based on LSI, not range chemistry.
Water Quality is about disinfection and water clarity. It is about managing contaminants and keeping the water clean and safe. Traditional pool care relies on chlorine to do almost everything, and we disagree. We are different because we do not over-chlorinate water. We supplement chlorine because it can't do everything.
Balance and quality are two different concepts. We at Orenda teach how to keep both sides of water chemistry in harmony. Our strategy embraces (and obeys) the laws of physics and chemistry and treats water the way water wants to be treated. For some reason, that's contrarian to the pool industry.
We don't want to be contrarian. We think the industry standards need to be updated to reflect reality. So let's dive deeper into what the traditional industry standards look like.
Range Chemistry (water balance)
| Factor | Range |
| pH | 7.2 - 7.8, ideally 7.4 - 7.6 |
| Total Alkalinity (TA) | 80 - 120 ppm |
| Calcium Hardness (CH) | 200 - 400 ppm |
| Total Dissolved Solids (TDS) | < 1500 ppm above tap water |
| Cyanuric Acid (CYA) | 30-50 ppm |
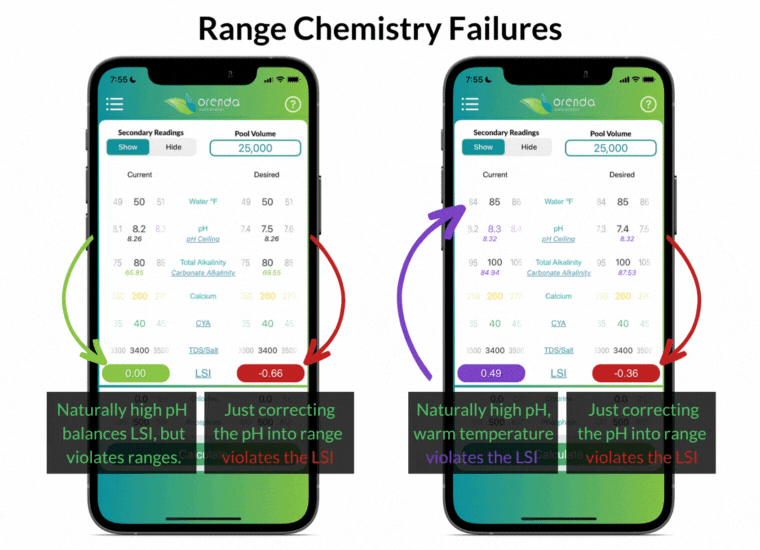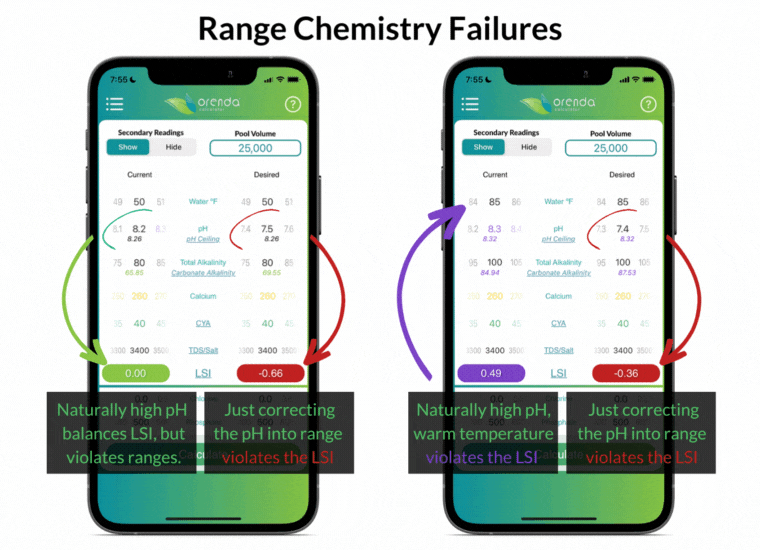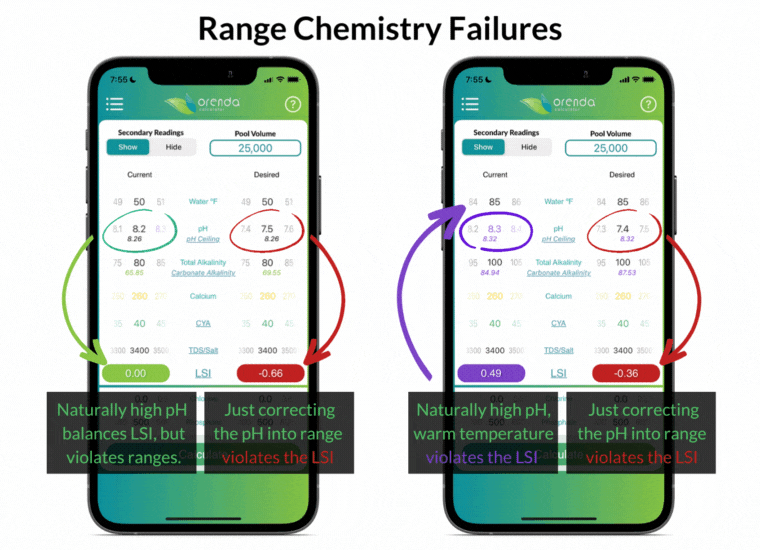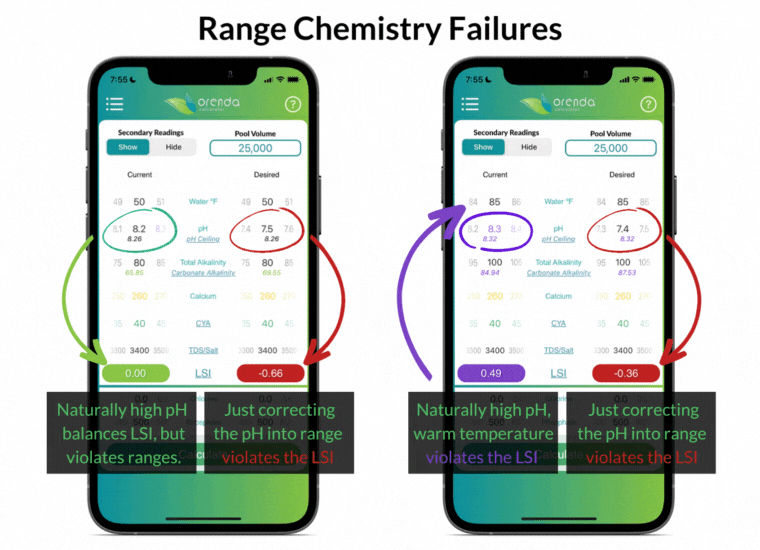
Simply "correcting" the pH back into textbook range violates the LSI in these examples. And what about the pH naturally rising, thanks to the loss of CO2? Furthermore, notice that the ranges above do not factor in water temperature at all.
The industry-standard ranges are the same for all pools, regardless of location and climate. Do swimming pools in Phoenix and Philadelphia have the exact same needs? The same rainfall, climate, and water temperature swings? Range chemistry standards ignore those factors in an effort to make things easier, as if they don't matter. But they do matter!
The effort to make things easier actually made water chemistry more complex. Instead of working toward an overall LSI balance, pool owners and operators must try to balance different individual ranges without regard to how they affect each other. We disagree.
For instance, having total alkalinity of 80-120 ppm is going to be a source of rising pH in most pools, so the pH will be out of range in a matter of days (or even hours in some pools).
This is the crux of why we differ from industry standards on water balance. We prioritize the Langelier Saturation Index (LSI), not range chemistry. Or, as we say, LSI first, range chemistry second.
It's not that the ranges are useless; it's that they don't always apply to every pool every time.
The LSI, however, is universal. The LSI is the holistic view of water balance, and it accounts for things like water temperature. Range chemistry does not. Individual chemistry factors like pH and total alkalinity matter, but they matter within the context of the LSI as a whole. Listen to our Rule Your Pool Podcast episode below for more on this:
Now let's explore how deep the range chemistry paradigm goes.
What's based on range chemistry?
Range chemistry is the basis for industry standards at all levels.
Industry Education and Textbooks
Perhaps it all boils down to education. At its core, industry certifications and water chemistry education have been based on range chemistry for decades. In the United States, the Certified Pool Operator (CPO) program is based on range chemistry. To their credit, the CPO class does mention the LSI, but not its importance. So the LSI is easily forgotten and overlooked in practice. In the United Kingdom, the Pool Water Treatment Advisory Group (PWTAG)'s water chemistry standards are also built upon range chemistry.
Textbooks are the same way. Many textbooks don't even mention the LSI, nor any other saturation index.3 Just range chemistry. And many of the ranges are arbitrary at best.
For instance, can anyone tell us why TDS needs to be less than 1500 ppm above tap water TDS? We have yet to find chemistry evidence to justify such a parameter (in fact, the compelling evidence suggests otherwise). Especially considering saltwater pools need a minimum of 3000 ppm salinity for the salt chlorine generator to work. So for salt pools, is it just 1500 ppm above the salt level? Or is it 1500 above the salt and the tap water? It's never specified.
Do you see the flawed logic here?
And how about Cyanuric Acid (CYA)? We at Orenda recommend staying below 50 ppm CYA, but some sources say it's fine to go as high as 100 ppm. Some manufacturers even recommend 60-80 ppm CYA for their salt systems to perform optimally. But what about how this elevated level of CYA impacts the LSI? And more importantly, what that excess CYA is doing to chlorination and water quality? If those factors were taken into consideration, industry standards would not be recommending 100 ppm CYA.
Now that's not to say that some pools cannot have 100+ CYA, because tens of thousands of pools in Arizona alone do. But are they LSI balanced? Rarely. And they are almost certainly over-chlorinated (but we'll discuss chlorination in the next section).
Related: Minimal CYA (Pillar 4)
We could go on and on. The consequences of teaching range chemistry ripple throughout the industry. For example...
Manufacturer Warranties
Okay, upfront, we have not read every product's warranty information. But during research for this article, we've read enough to see a clear pattern. Almost every manufacturer is using the ranges outlined above, or very close to them. If you violate range chemistry–as outlined in a manufacturer's warranty documents–you will void the warranty.
Some products have slightly different ranges, which leads to conflicts even within the same pool! For example, the ranges listed in salt chlorine generator warranties sometimes conflict with heater warranties. Or plaster surface warranties. Or vinyl liner and fiberglass pool warranties.
Many warranty documents insist water chemistry meets "standards established by [xyz trade association]". This has been seen as a safe, liability-limiting way of handling the chemistry aspects of warranties. But if you look at these ranges with a critical eye (like we have), you'll find they are NOT always a good idea.
Wouldn't it be easier (and better) to have warranties based on the LSI? The LSI applies to every pool in the world, universally. It would no longer matter what type of surface you have, or what type of equipment. And the water temperature is accounted for. LSI balance protects all surfaces and equipment throughout the year, and it takes all the factors into account simultaneously.
This, of course, should be done with a practical understanding of how these individual chemistries will change over time. A factor like pH should have minimums established to protect equipment while relaxing the maximum to allow for natural aeration and pH rising. There are too many specific situations to write about here in this blog, but if there are any manufacturers reading this who want help with the chemistry portions of their warranty documents, feel free to contact us. We are happy to help you with this.
But alas, industry standards still rely on outdated ranges. We hope this article ignites change.
Health Codes
Health codes and standards are also based on range chemistry. Particularly for pH, because of pH's impact on chlorine strength and speed (contact times, or CT values). When we talk about chlorine strength and speed, health departments care about the percentage of the killing form of chlorine, Hypochlorous Acid (%HOCl). The higher the percentage of HOCl, the faster your chlorine can kill germs and algae. This is the chart health departments refer to (on the left):
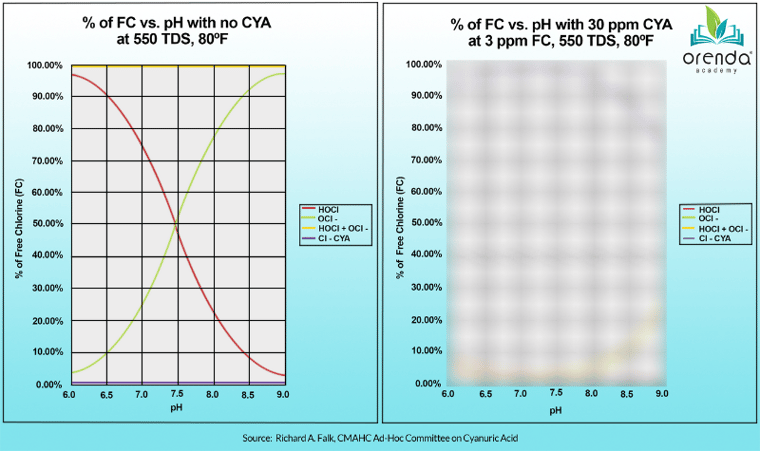
The lower the pH, the higher the %HOCl relative to its much weaker counterpart, Hypochlorite ion (OCl-). HOCl is the red line, and OCl- is the green line. But this chart is only true for pools with zero CYA in them, like indoor pools. The moment you introduce CYA to your water, you no longer get to use this chart. It doesn't apply to your water anymore. The chart for water with CYA in it looks more like the one on the right:
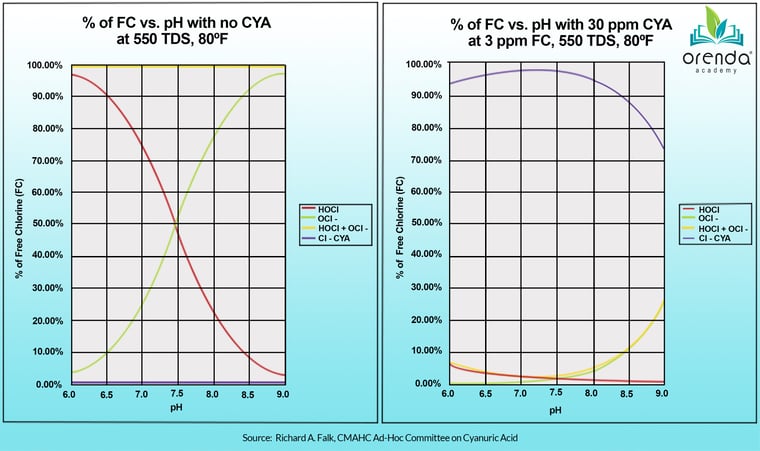
Where's the red line now?
What this tells us is when CYA is in your water, pH is not commanding the strength and speed of your chlorine nearly at all. We elaborate much more on this here and in this podcast episode:
Health departments still use the left chart, even if you have CYA in your water. Why? Because the industry standards have not yet been updated to reflect the impact CYA has on chlorination. This must change for the sake of swimmers in public pools.
In particular, pH ranges are overdue for a change, especially in outdoor commercial pools with CYA. In our opinion, chlorine requirements should be based on what actually impacts contact times (CT) for disinfection. Namely, that's the free chlorine to CYA ratio (FC:CYA).4 Read the research yourself.5
It will be a huge win for the industry when health codes are updated to relax pH parameters to allow for a higher pH and adopt CYA limitations.6 Both water balance and water quality will be easier to manage, and it will be safer for bathers to swim in. Everybody wins.
The unfortunate reality, whether we like it or not, is that health departments can still shut pools down for doing exactly what we just described. If you violate the pH or alkalinity ranges, there are consequences. So until the standards change and the health departments adopt those changes, commercial pools are stuck obeying a system that forces more acid and bicarb use (and for a worse outcome for bathers).
Pool Store Testing Software
When you get your water tested at a pool store, you are likely to get a printout of recommendations for what you need to do and the products needed to do it. These automated recommendations are based on range chemistry that may not apply to your pool. An Orenda prescription for your pool looks very different than what you will get from a pool store.
What's funny is that some of these pool store printouts actually do measure the LSI, yet the ranges could still be marked as out of balance even if the LSI is fine. This tells us that the LSI is more of a bonus read-out than a primary focus for these software programs.
Conventional Wisdom and Habits
Range chemistry is the basis for decades upon decades of bad habits. Old-school pool care was built on range chemistry. We get it, but we also find it odd that pool pros have put up with it for so long. Has it ever worked?
Be honest with yourself for a moment. With 80-120 ppm total alkalinity, have you ever been able to hold 7.4-7.6 pH for a week (without an auto cover, trichlor feeder, acid feeder, or borates)? Have you ever been able to keep all your ranges in line for seven days so that when you return, everything–including pH–is exactly where it's supposed to be?
Ever?
No? Okay cool, because neither have we. In fact, nobody has. Nobody can. These chemistry ranges are physically impossible, and we know that because of physics.7 In other words, traditional range chemistry violates physics. Unless you're constantly manipulating pH with an acid or CO2 feeder, your water violates range chemistry every week anyway.
So let's not pretend that by following range chemistry, we've got everything–including pH–under control. None of us ever did, and none of us ever will.
Water balance, the Orenda way
Since water only cares about balance, and balance is measured using the LSI, the LSI is our top priority.
Individual chemistry factors should be decided within the context of the LSI. For instance, you probably need less than 80 ppm total alkalinity in order to stay LSI balanced for a week in the summer. You may also need more than 400 ppm calcium hardness, depending on where your pool is. And you can bet on the pH naturally rising up each week, so be prepared for that too.
Just because our strategy is different does not mean it is more complicated. It's actually quite simple: contain pH so that LSI balance is maintained year-round.
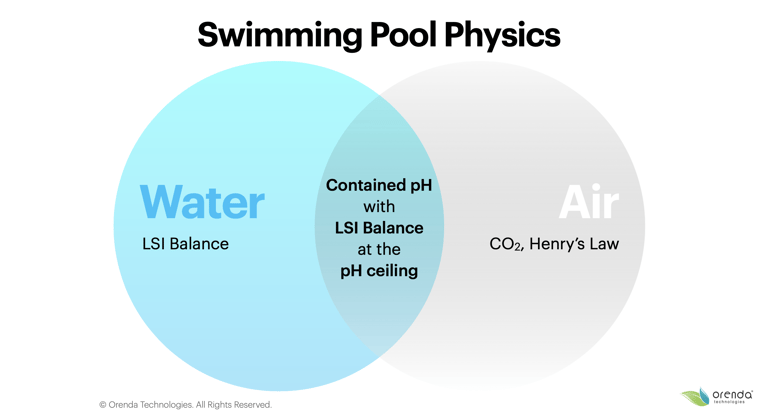
Stay in the middle of the Venn diagram. You can limit the pH ceiling by having less carbonate alkalinity (and more calcium hardness to offset the lower alkalinity on the LSI). If you do it right, your pH is contained, and your LSI should be balanced 24/7. This means you are able to know for sure that your LSI will not get out of balance during the week. Range chemistry does not enable that to happen...in fact, it virtually guarantees you will be out of balance each week.
It may be necessary to violate a few traditional range chemistry parameters to pull this off, but the water will be balanced. We assure you that water has never read the textbooks.
Orenda's advice doesn't always contradict these standards. In fact, oftentimes, balanced range chemistry is also balanced LSI chemistry. However, as the examples show, that is not always the case, which is why we recommend LSI first, and range chemistry second.
Over-chlorination (water quality)

Pool treated weekly with enzymes CV-600
Now let's shift gears and talk about the other side of water chemistry. Water quality is about cleanliness, disinfection, oxidation, and generally keeping the water safe to swim in. Standard practice in the pool business is to chlorinate heavily to keep the water clean and safe. And sure, that sometimes works, but chlorine is not designed to handle everything it's tasked with.
Chlorine demand
Let's look at chlorine demand as two categories of contaminants: living and non-living. The living contaminants are the sanitizer demand, and the non-living contaminants are the oxidant demand.
Sanitizer demand
 Sanitization/disinfection is chlorine's primary job in water. Chlorine easily kills germs, viruses, and other infectious pathogens. It also kills non-infectious microorganisms like algae. Overall, unless you have an algae bloom, sanitizer demand is a small percentage of the overall chlorine demand. And that's a good thing because we don't want any pathogens in the water in the first place.
Sanitization/disinfection is chlorine's primary job in water. Chlorine easily kills germs, viruses, and other infectious pathogens. It also kills non-infectious microorganisms like algae. Overall, unless you have an algae bloom, sanitizer demand is a small percentage of the overall chlorine demand. And that's a good thing because we don't want any pathogens in the water in the first place.
The vast majority of chlorine demand is non-living contaminants that chlorine must oxidize.
Oxidant demand
There are three categories of non-living oxidants in swimming pool water: metals, nitrogen compounds, and non-living organics. See the pie chart below:
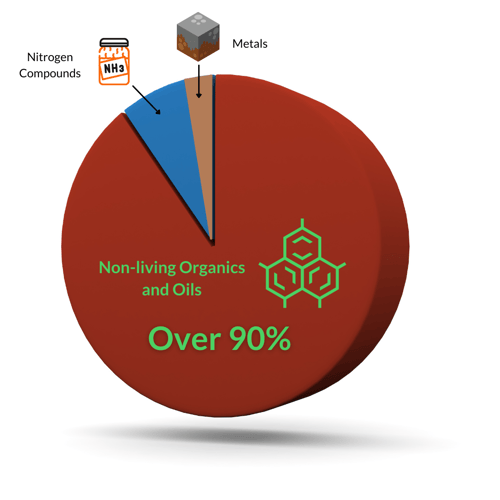
Metals are the first thing to be oxidized because they are the easiest for chlorine to oxidize. Thankfully, the amount of metals is usually very low, unless you have high metal content in your tap water. Iron, in particular, is the easiest thing for chlorine to oxidize. It turns reddish-brown and eventually leads to staining issues.
The next category is nitrogen. Residential pools do not often struggle with nitrogen compounds because their bather loads are relatively small, and the sources of nitrogen are limited. But nitrogen compounds do get introduced from decaying organics, fertilizers, ammonia-based cleaning products, bird droppings, and swimmers peeing.
The most common sources of nitrogen in residential pools are ammonia-based algaecides. Anything that contains words like "ammonium" or "quat" (short for quaternary ammonia) will contain ammonia in some form or another. It takes a lot of chlorine to oxidize and remove ammonia from the water via the breakpoint chlorination process.
 Finally, the lion's share of oxidant demand (and chlorine demand in general) is non-living organics. These include natural organic material like decaying leaves and grass clippings, soils, pollen, but also natural body waste like sweat and body oils. That said, natural organics are somewhat simple for chlorine to oxidize compared to man-made organics. We're talking bather products like cosmetics, lotions, soap, deodorant, tanning oils, and perhaps the biggest of them all, sunscreen.
Finally, the lion's share of oxidant demand (and chlorine demand in general) is non-living organics. These include natural organic material like decaying leaves and grass clippings, soils, pollen, but also natural body waste like sweat and body oils. That said, natural organics are somewhat simple for chlorine to oxidize compared to man-made organics. We're talking bather products like cosmetics, lotions, soap, deodorant, tanning oils, and perhaps the biggest of them all, sunscreen.
Oils and organics float, while dirt and debris sink. When they stick to one another, they tend to stay suspended in water for longer, leading to cloudy water. See the before-and-after of the same pool after CV-600 enzymes removed the non-living organics from the water:
Chlorine was designed to kill microorganisms, not to oxidize sunscreen and oils. Yet the pool industry standard has been to over-chlorinate pools to keep them clean and clear. In our experience, simply throwing more chlorine at the problem is not an effective way of managing it. If it were, pools would never have scum lines on their tile, and the water would always be clear.
Furthermore, chlorination leaves behind byproducts, depending on what it oxidizes.
Chlorine byproducts
Chlorine is the most popular sanitizer for two reasons: it's a very effective, naturally-occurring sanitizer (we have HOCl in our white blood cells, for instance8), and its byproducts from killing germs are generally harmless.
Unfortunately, the byproducts of oxidizing nitrogen compounds and certain organics are not harmless. There is a growing body of research about chloramines and chloro-organic compounds, which all fall under the umbrella of disinfection byproducts (DBPs). You can measure chloramines with combined chlorine, which you do not want in your water at all. We encourage you to do the research yourself, and you can start with this collection of articles. In summary, the less DBPs in your water, the better.
There are two main ways to minimize DBP production:
- Reduce the source of the contamination (i.e. swimmers showering before jumping in the pool, encourage swimmers to not pee in the pool, and avoid using ammonia-based algaecides), and
- Supplement chlorine against the oxidant demand (Pillar 2).
Water Quality, the Orenda way
%20-%20Edited.png?width=760&height=428&name=Elite_CV600_SQ_Clean_A7307715%20(1)%20-%20Edited.png) We do not over-chlorinate pools; we supplement chlorine appropriately. Chlorine alone was not designed to handle all the contaminants in water. So we use it for its primary purpose of sanitizing, and supplement it to help address the oxidant demand. The result is improved chlorine efficiency, cleaner and safer water, and less chlorine demand.
We do not over-chlorinate pools; we supplement chlorine appropriately. Chlorine alone was not designed to handle all the contaminants in water. So we use it for its primary purpose of sanitizing, and supplement it to help address the oxidant demand. The result is improved chlorine efficiency, cleaner and safer water, and less chlorine demand.
We can supplement chlorine with enzymes, a secondary system (Ozone, UV, or AOP), or both.
In either case, we reduce the burden on chlorine so it can focus on what it does best: kill.
Conclusion
In the two disciplines of water chemistry (water balance and water quality), the Orenda Program differs from industry standards.
In water balance, our priority is the LSI, not individual ranges (range chemistry). Ranges could be okay if they are in the context of the LSI, but that would mean ranges are different wherever you go...but the LSI is universal. We are openly trying to shift the pool industry away from the outdated range chemistry paradigm and onto the LSI. That's why we have the Orenda Calculator™, Orenda Academy™, this blog and the Rule Your Pool Podcast. Everything from textbooks, pool store software, warranty information, and old habits will benefit from this change. Pool owners especially will benefit.
In water quality, we do not rely on chlorine to do everything, therefore we do not over-chlorinate pools. Instead, we properly chlorinate and supplement chlorine against the oxidant demand. We decide to remove as much contamination from water as possible. In our program, simpler chemistry makes for cleaner, safer water.
We could have added several other ways we differ from industry standards, but we felt like these two are enough to get the point across. So if anyone questions why your Orenda pool is managed differently than industry standards, now you know. We hope this article was helpful for you, and feel free to share it.
1 The pool industry has had many industry organizations over the years. There was NSPI, APSP, NSPF, and more. Several of them have merged into what is now known as the Pool and Hot Tub Alliance (PHTA). There are also trade-specific organizations like the National Plasterers Council (NPC), amongst others. Each of these organizations gives validity to range chemistry by publishing their own recommended ranges. And the ranges are not always the same in every trade group. And if you look internationally, there are other swimming pool industry standards that also use ranges, but they are different from those you find in America. The point is, who is right? And do you really think pools in completely different climates need to be managed the exact same way? We don't.
2 Water Quality Fact Sheets. Updated 2022. https://phta.org.
3 There are other saturation indices that are similar to LSI. Ryznar Stability Index (RSI), Hamilton Index, and some sources refer to the LSI as the Calcite Saturation Index (CSI), though that's the same exact formula. All of them are simlar, so if you're LSI-balanced, you're most likely balanced on the Hamilton and Ryznar indices too. We chose to pick the most universally recognized LSI for the Orenda Calculator.
4 Pickens, Stanley R., Ph.D. (2017). Relative Effects of pH and Cyanurate on Disinfection. White paper published on Poolhelp.com
5 Pulsar Systems. The Effect of Cyanuric Acid on Disinfection. White paper published on Pulsarsystems.net
6 We would love to see pH parameters relaxed to allow for higher pH. Ideally up to 8.2, but we'll settle for 8.0 in commercial water. Just the difference between 7.8 and 8.0 in chlorinated swimming pools can translate to massive cost savings on acid and sodium bicarb alone. And it will not affect chlorine performance or efficiency as long as the free chlorine to cyanuric acid ratio (FC:CYA) is in line.
7 Violating laws of physics is where the term "physically impossible" comes from.
8 Block, M. S., & Rowan, B. G. (2020). Hypochlorous Acid: A Review. Journal of oral and maxillofacial surgery: Official journal of the American Association of Oral and Maxillofacial Surgeons, 78(9), 1461–1466. https://doi.org/10.1016/j.joms.2020.06.029
