Swimming Pool Covers and Water Chemistry

Swimming pool covers impact water chemistry in different ways. This article discusses each type of pool cover and how they affect water chemistry. Perhaps most importantly, we will discuss how the type of pool cover you have determines how the pool should be winterized and closed.
Covered in this article:
- Different types of swimming pool covers
- In-season covers
- solar pool cover
- automatic pool cover
- Safety covers
- mesh pool cover
- solid or automatic pool cover
- In-season covers
- Pool winterization chemistry
- Mesh cover (or no cover)
- dilution
- rising pH
- how to winterize with a mesh cover (or no cover)
- Solid cover
- no dilution
- pH suppression
- how to winterize with a solid cover
- Mesh cover (or no cover)
- Conclusion
Different types of swimming pool covers
There are two purposes for using pool covers. Covers used in-season, and those used for closing the pool for the winter. Then there are variations of both that we are about to discuss.
In-season pool covers
Pool covers used in-season are generally thermal pool covers designed to insulate the pool from losing too much heat. The purpose is two-fold: save energy by reducing heat loss, and save water by minimizing evaporation. For instance, indoor commercial pools can significantly benefit from a thermal cover to reduce evaporation loss, saving the pool dehumidification system a tremendous amount of energy. Thermal covers, also called thermal blankets, are sometimes foam insulated and float on the top of the water to hold in the most heat.
Solar pool covers

A popular type of thermal cover is called a solar cover. Solar covers are usually translucent plastic with air bubbles, so they float at the surface of the water. They allow sunlight to heat the water beneath the cover while simultaneously holding most of that heat in the water. They also prevent most evaporation from occurring. Solar covers (also called solar blankets) are also used in indoor pools for the same reasons. They are usually stored on large reels either mounted to a wall or on wheels to be moved around.
Solar covers cannot support a person's weight (or pet), and therefore cannot be used as safety covers. If you were to step on a solar cover, you would fall right in. We can confirm this from personal experience. Competitive swimmers don't always make the best choices.
Automatic Pool Covers

Photo courtesy of Latham Pool Products
Rectangular pools (and free form pools if they have a recessed deck) sometimes have built-in solid covers, which are not the same as a solar cover (but sometimes they can be). Usually, automatic covers are made of reinforced vinyl that rolls out along two tracks so that the entire cover can be opened and closed with a permanently mounted controller in clear view of the pool. Automated pool covers are great for both in-season and winterization use because they double as a safety cover. While it's not recommended for a person to walk across an automatic cover, they should be able to withstand the weight in the case of an emergency.
Pool Safety Covers
 If your pool was closed down and winterized for the off-season, there's a good chance you have a safety cover. Depending on where you live, safety covers may be required.1 A safety cover is anchored to the deck using a bunch of anchor screws around the perimeter. These anchors are recessed into the deck during the season, so nobody hits them with bare feet. When it's time to close, a drill or special tool can loosen these bolts and raise them about a half-inch; just enough for the spring-tension hooks of the cover to lock.
If your pool was closed down and winterized for the off-season, there's a good chance you have a safety cover. Depending on where you live, safety covers may be required.1 A safety cover is anchored to the deck using a bunch of anchor screws around the perimeter. These anchors are recessed into the deck during the season, so nobody hits them with bare feet. When it's time to close, a drill or special tool can loosen these bolts and raise them about a half-inch; just enough for the spring-tension hooks of the cover to lock.
Safety covers are either mesh or solid. We'll discuss how each impact winterization chemistry differently in the next section. Regardless if you have a mesh or solid safety cover, they are made to prevent people or animals from falling into the pool and drowning. Many brands boast the ability to hold the weight of a car or an elephant. As a general rule, safety covers are strong by design.

Pool winterization chemistry
The name of the game for winterization is to maintain LSI balance during the coldest part of the year. If you're able to maintain LSI balance in the dead of winter, the likelihood of problems like calcite crystals or winter dust is very low. For vinyl liner or fiberglass pools, problems like fading and fiberglass "chalking" can also be prevented because those issues tend to occur more during the winter. Yes, most pool damage occurs during the winter, thanks to cold water lowering the LSI.
Related: Pool chemistry during the winter
The chemistry strategy you should use when winterizing depends more on the type of safety cover than the type of surface. In other words, you would winterize a vinyl liner or fiberglass pool the same way you would a gunite pool. It's the type of cover on the pool that changes the strategy.
Mesh cover (or no cover)

Photo Courtesy of Meyco Covers
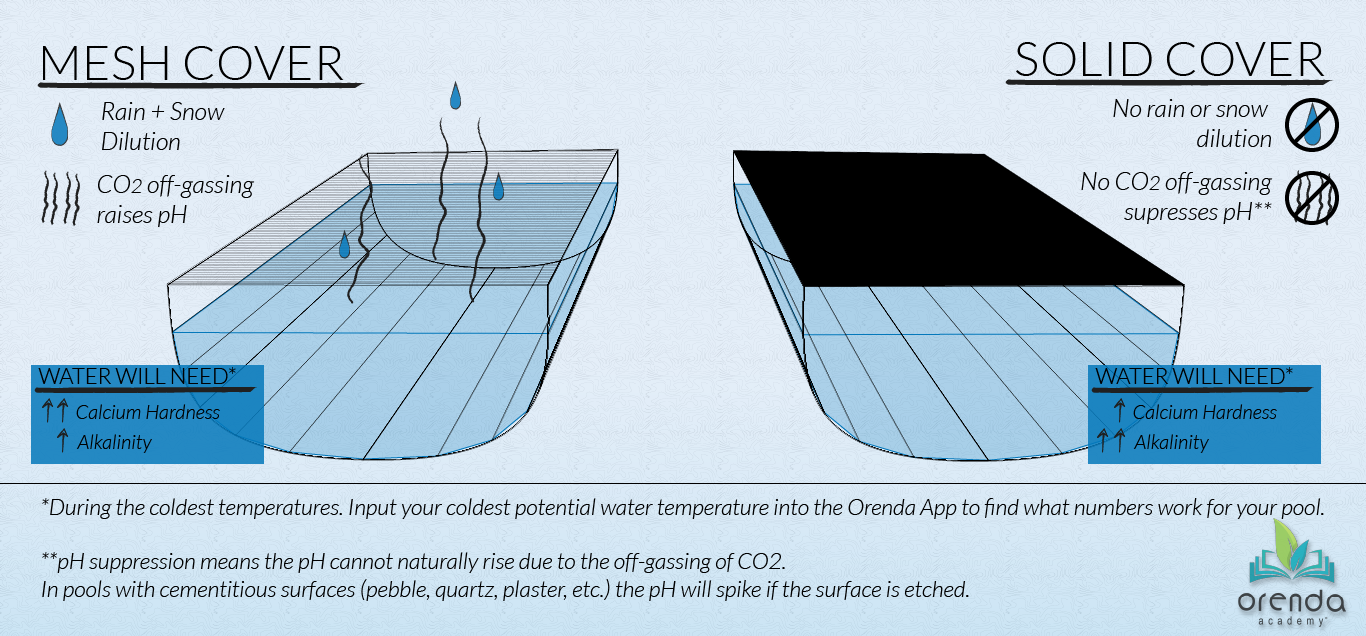
Mesh safety covers allow both water and air to pass through. So they allow rain and snow to get into the pool and allow gas–specifically carbon dioxide–to escape. Your winterization strategy with a mesh-covered pool needs to account for 1) dilution and 2) rising pH.
Dilution
Rain and snow have zero calcium hardness, cyanuric acid, salt, and TDS. It's distilled water, so rainwater is extremely low on the LSI, diluting your pool chemistry over several months. Calcium hardness, CYA, and TDS will all decrease over the winter as a result. That's not all bad news, of course, since reducing CYA helps your chemistry in more ways than one. But are you reducing calcium? That can be problematic during the winter since calcium is your best friend for maintaining LSI balance in cold water.
Rising pH
Since pH will reach its ceiling at about 8.2 over time, you can afford to ignore pH when you winterize unless you are still circulating and feeding acidic chlorine like trichlor. If you are shutting the system off and are no longer chlorinating, ignore pH and let it rise naturally up to its pH ceiling of about 8.2.
How to winterize with a mesh cover (or no cover)
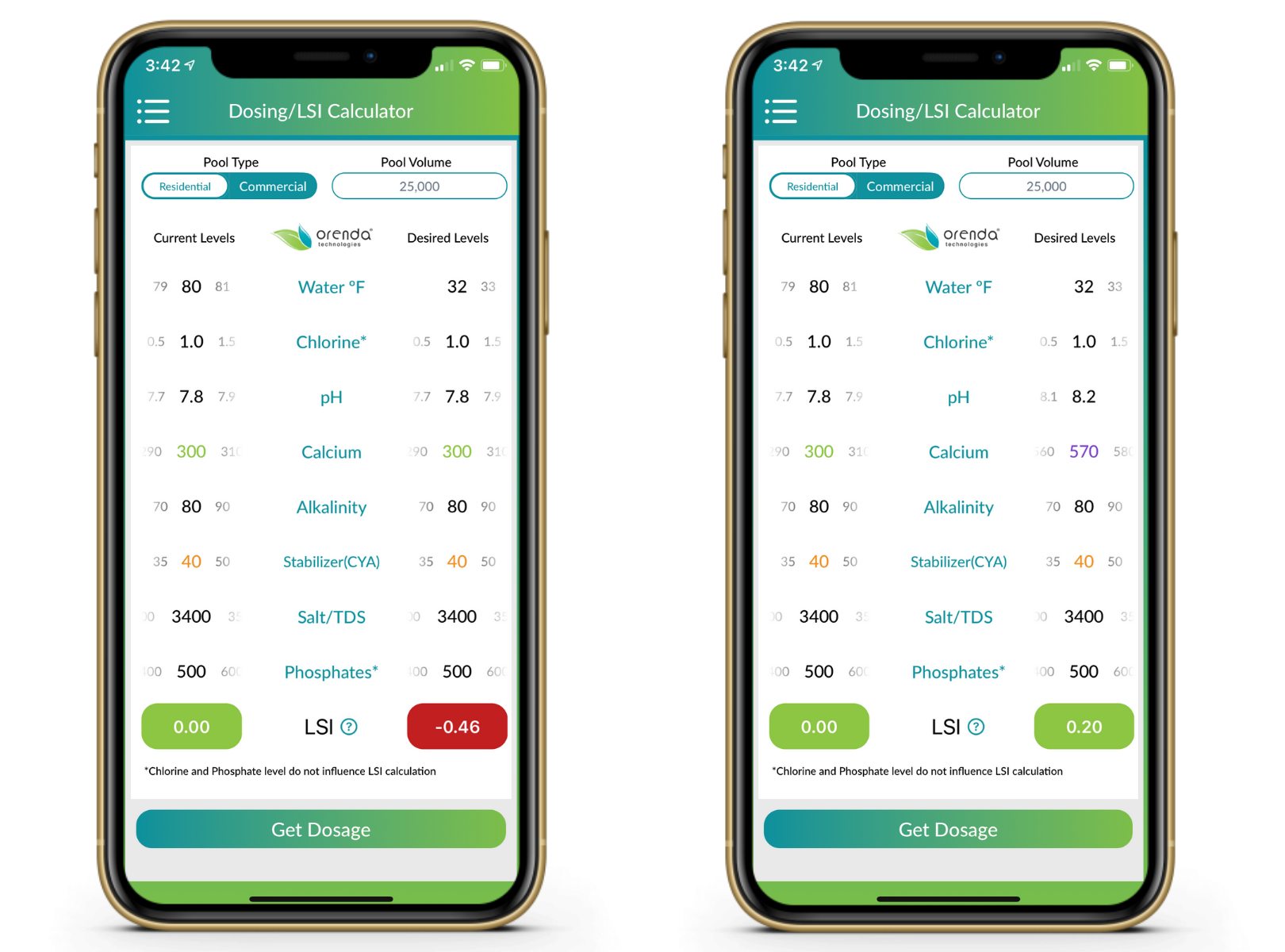 You'll need to load the pool up with more calcium than you think because dilution will bring it back down. We used to recommend a minimum of 400 ppm calcium if your pool freezes, but we are finding that mesh pools need more than 500 ppm calcium to combat dilution. Then, ignore pH and let it rise to 8.2, drop the temperature down to its lowest point predictable on the Orenda app, and see how your LSI looks. If it's not quite balanced yet, you might need more alkalinity. Play around with the app or contact us for coaching.
You'll need to load the pool up with more calcium than you think because dilution will bring it back down. We used to recommend a minimum of 400 ppm calcium if your pool freezes, but we are finding that mesh pools need more than 500 ppm calcium to combat dilution. Then, ignore pH and let it rise to 8.2, drop the temperature down to its lowest point predictable on the Orenda app, and see how your LSI looks. If it's not quite balanced yet, you might need more alkalinity. Play around with the app or contact us for coaching.
Solid cover

Whether installed at the end of the season or automatic, a solid safety cover impacts chemistry in two ways: 1) it prevents rain and snow from getting into the pool and diluting it, and 2) it prevents carbon dioxide from escaping. Meaning the pH is unable to rise naturally to its 'ceiling'. Both of these characteristics change the protocol for winterizing when compared to a mesh or uncovered pool.
No dilution
Because rain and snow cannot get into the pool to dilute the calcium hardness, TDS, and CYA, the chemistry you close with is pretty much the chemistry the pool is stuck with for the winter. This can be a good thing if you're trying to maintain higher levels of calcium hardness, but it's bad if you depend on rain to help dilute out negative things like cyanuric acid (CYA). No dilution means you need to close with enough calcium and alkalinity on the closing day to survive the coldest water temperatures. That is, of course, unless you continue pool service through the winter so that chemistry can be adjusted each month during the offseason.
pH suppression
Because CO2 cannot escape, it will not equalize with the atmosphere above the pool. Your pH can only rise so high (unless the cover is loose enough to let air sneak out from under it). Realistically, the pH you close with is the pH your water will remain at unless it's a gunite pool with a cementitious surface that etches.2 In other words, with a solid cover, pH can only rise if it's forced, because you have taken away the natural means for it to rise on its own (Henry's Law), causing pH suppression.
How to winterize with a solid pool cover
You won't need quite as much calcium hardness with a solid cover because you won't have dilution. But then again, your pH won't be able to rise to its ceiling either. So winterizing a pool–regardless of surface type–with a solid cover means chemistry that can maintain LSI balance throughout the winter. Higher calcium hardness is good, but instead of depending on a climbing pH, you might need more alkalinity in a solid pool. Again, play with the Orenda app to see what works for you, but you will want roughly 400-500 ppm calcium hardness, and about 100-150 ppm alkalinity in round numbers. These should not both be added on closing day, because the pool will cloud up like crazy.
Related Procedure: How to Winterize a Pool the Orenda Way
Conclusion
The type of cover matters more for winterization than you might think. All types of pools need LSI balance throughout the winter, so the surface does not matter in that sense. That being said, the consequences of unbalanced winterization chemistry are different. Cementitious finishes get calcite crystals, discolorations, and/or winter dust, accompanied by a major pH spike. Vinyl liners tend to fade and lose pigment. Fiberglass pool gel coats deteriorate and get oxidized, which turns white–a condition we call "chalking." All of these issues can be prevented by proper winterization practices, anchored with an LSI strategy that is right for your pool based on your local climate and the type of pool cover you use. If you have more questions, comment below or contact us. We hope you found this information valuable.
1 Exclusion to this rule depends on your city/county's health and safety codes. For instance, a commercial community pool may not need to be covered if it has appropriate fencing and locks to prevent people from getting into the pool area and potentially falling in. Without such fencing and barricades, safety covers are usually required.
2 Because CO2 is contained under the solid cover, Henry's Law, finds equilibrium in the space given, as opposed to the general atmosphere. The smaller space limits CO2 off-gassing; therefore, the pH will not naturally rise up much at all. Etching, however, draws 12.6 pH calcium hydroxide, which spikes the pH well above the pH ceiling.
