Chlorine, pH and Cyanuric Acid Relationships

It is widely known that pH controls the strength and efficacy of chlorine. And that's true, except when Cyanuric Acid (CYA) is in the water. CYA changes the entire dynamic, and that's what we discuss in this article.
Covered in this article:
- Defining "Chlorine Strength"
- Contact Time (CT)
- Concentration of Hypochlorous Acid (%HOCl)
- Cyanuric Acid and Hypochlorous Acid (HOCl)
- Stabilized chlorine vs. non-stabilized chlorine
- The free chlorine to cyanuric acid ratio (FC:CYA)
- Chasing pH is a losing strategy
- Conclusion
Defining Chlorine Strength
Let's first define the term chlorine strength. The word strength is a misnomer. Chlorine disinfection is all about killing speed. How fast can a given concentration of chlorine kill certain pathogens and other contaminants like algae? This is quantified using a metric called Contact Time (CT).
Contact Time (CT)
Germs and other harmful pathogens have known contact time (CT) values. CT values tell us how much time it takes for a given amount of active free chlorine to kill a pathogen.1
A CT value of 10 means 1 ppm of free chlorine will kill the pathogen in 10 minutes, 5 ppm takes 2 minutes, or 10 ppm takes 1 minute. It's time(minutes) multiplied by the free chlorine(ppm or mg/L).
CT = Sanitizer concentration(ppm or mg/L) x Time(minutes)
CT = Free chlorine(ppm or mg/L) x Time(minutes)
To keep water safe, clean, and clear, a higher percentage of the active form of chlorine, Hypochlorous Acid (%HOCl), is desired. The higher the %HOCl, the better and faster the free chlorine can kill and oxidize contaminants.
In the pool industry, chlorine strength refers to the %HOCl. The amount of free chlorine is important too. We'll expand on this in a moment.
Concentration of Hypochlorous Acid (%HOCl)
Ultimately, the concentration of HOCl matters most for disinfecting germs and sanitizing other microorganisms (like algae). The percentage of HOCl alone is not enough, because if there is hardly any chlorine in the pool, a high percentage of very little is still very little.
Conversely, elevated free chlorine levels may also have a small concentration of HOCl in the water, depending on the pH and the amount of Cyanuric Acid (CYA). This article summarizes the abundant research about how CYA impacts chlorine, and in particular, the concentration of HOCl.2
There are three takeaways from this article:
- CYA substantially reduces the concentration of HOCl in the water, which slows chlorine down.
- When CYA is present, the impact pH has on %HOCl is minimal, if not negligible. The ratio of free chlorine to CYA (FC:CYA) is more significant than pH in terms of attaining the desired concentration of HOCl required to kill pathogens and algae.
- Based on (1) and (2) above, maintaining minimal CYA is more important for chlorine disinfection than maintaining a specific pH.3,4
Cyanuric Acid and Hypochlorous Acid (HOCl)

We began this article by defining chlorine strength as the concentration of HOCl in the water (%HOCl). The higher the %HOCl, the faster chlorine can kill and oxidize. And since killing pathogens is about contact time (CT), speed is the name of the game. So what does CYA do to the %HOCl?
CYA substantially reduces the concentration of HOCl in the water and therefore slows chlorine down.2,3,5,6
HOCl binds with CYA in water in a substitution reaction. The nitrogen bonds that hold chlorine are weak, allowing chlorine to be released from CYA to do its job. This process involves several chemical reactions and interactions:
1. HOCl Formation:
When chlorine gas (Cl₂) dissolves in water, it undergoes a series of reactions to form hypochlorous acid (HOCl) and hydrochloric acid (HCl):
Cl2 + H2O ⇄ HOCl + HCl
Chlorine + Water ⇄ Hypochlorous Acid + Hydrochloric acid
Hypochlorous acid is the active disinfectant species responsible for killing microorganisms. In a non-stabilized pool (meaning no CYA), the pH determines the percentage of active HOCl compared to its slower and weaker conjugate base, Hypochlorite Ion (OCl-), based on Hydrogen concentration:
HOCl ⇄ H+ + OCl-
Hypochlorous Acid ⇄ Hydrogen ion + Hypochlorite ion
The graph looks like this:

2. Binding with Cyanuric Acid:
In the presence of HOCl, CYA forms chlorinated isocyanurates through a substitution reaction:
3HOCl + CYA ⇌ Cl₃CYA + 3H₂O
3 Hypochlorous Acids + Cyanuric Acid ⇌ Trichloroisocyanuric Acid + 3 Waters
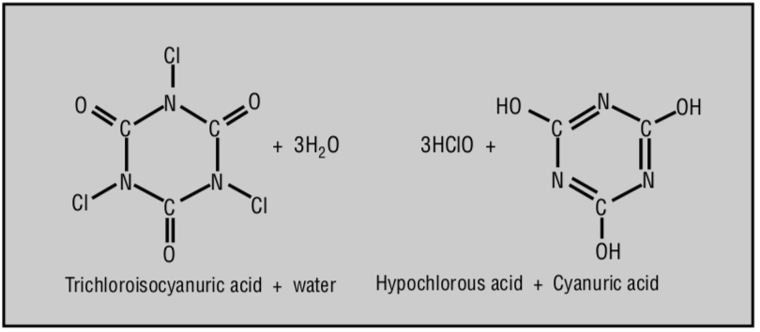
This reaction produces chlorinated isocyanurates, such as trichloroisocyanuric acid (Cl₃CYA), which are more stable in sunlight than either HOCl or OCl- on their own.
3. Chlorine is released to kill or oxidize contaminants:Chlorinated isocyanurates, particularly trichloroisocyanuric acid, release HOCl in water:
Cl₃CYA + H₂O → HOCl + CYA
Trichloroisocyanuric Acid + water → Hypochlorous Acid + Cyanuric Acid
The released HOCl acts as a potent disinfectant, oxidizing cellular components of microorganisms, disrupting their metabolic processes, and ultimately leading to cell death.
To recap, chlorine in water forms HOCl, which quickly binds to CYA to form chlorinated isocyanurates that are resistant to sunlight degradation. HOCl is released from CYA when a contaminant needs to be killed or oxidized. This is the essence of chlorine stabilization.
Stabilized chlorine vs. non-stabilized chlorine
Looking at the previous chart, you can see why textbooks teach us that lower pH means stronger chlorine. And this is true when CYA is not in the water.
But we now know that CYA has a profound impact on the concentration of HOCl, rendering pH much less important for chlorine strength.5,6 To date, we have found over a dozen peer-reviewed scientific articles that all reiterate the same concept. Here's a quote from one of them, Edmondo Canelli, in 1974:
"In the absence of cyanuric acid, the pH value determines the chlorine activity, since it defines the relative concentration of the form HOCI in aqueous solutions of inorganic hypochlorites according to:
OCI- + H+ ⇌ HOCl
And since the germicidal activity of the acid form, HOCl is approximately 100 times that of the base form OCI-. However, the pH has no remarkable effect when cyanuric acid is present in the system.7
This information about CYA and chlorine has been known and published since 1974. It was just never widely adopted in the swimming pool industry. This is not new information. Yet when we speak about this topic, most people seem surprised and skeptical. To be fair, so were we. Until we saw the graph.
Strength of chlorine (%HOCl)
It helps to visually see the comparison between non-stabilized chlorine and stabilized chlorine. Below is a chart comparing the previous chart (with no CYA) to that same pool with 30 ppm CYA. The chart below comes from a study published by the Council for the Model Aquatic Health Code's ad hoc committee on cyanuric acid.6 We recreated it to make it look prettier and have color-coded lines on it. The data originally come from a study done in 1974 by J. O'Brien et.al.8

The red line is strong HOCl, and the blue line is weak OCl-. Compare the red line (%HOCl) on the left chart to the red line on the right chart. Where is the red line now?
As you can see, CYA has taken over pH's role in controlling the strength and speed of chlorine. The %HOCl plummets to below 3% at 7.5 pH. Compare that with 50% HOCl without stabilizer on the left chart.
What happened here is simple: chlorine has bound to CYA, which creates chloroisocyanurates (represented by the purple line, Cl-CYA on the chart). The green line is a combination of all the chlorine that is not bound to CYA. As you can see, the vast majority of chlorine is bound to CYA at 30 ppm. This chart tells us two things:
- First, just 30 ppm of CYA binds to the vast majority of chlorine, which protects it well from sunlight. The desire to have much higher ppm of CYA seems misguided, unless you have higher levels of free chlorine to go along with it (FC:CYA ratio), and
- Second, in the presence of CYA, most chlorine has to detach before sanitizing or oxidizing, which increases contact time (CT). In other words, CYA slows chlorine down.
There is virtually no difference in chlorine strength (%HOCl) between a pH of 7.0 and 9.0 when CYA is in the pool.
We know this information seems contrary to decades of what we have all been taught in the industry. It felt like a punch in the gut to us when we learned it. Most of us have spent years treating pools by trying to keep pH where we wanted it to be (7.4-7.6). In reality, the water itself did not want to be there at all (which we will elaborate on in a moment). The truth is, pH does not control chlorine strength in a stabilized pool; the free chlorine to cyanuric acid ratio does (FC:CYA).
The free chlorine to cyanuric acid ratio (FC:CYA)

Notice the effective HOCl concentration is virtually the same, provided you maintain the FC:CYA Ratio. Source: CMAHC Ad-Hoc Committee for Cyanuric Acid
Thanks to the CMAHC, the FC:CYA ratio will hopefully be adopted as the new standard in place of blanket CYA ppm ranges or free chlorine minimums. Effective disinfection is the name of the game, after all.
Since the FC:CYA ratio dictates the %HOCl, then it needs to be front and center on our minds as we operate swimming pools. This is why the Model Aquatic Health Code (MAHC) recommends a maximum of just 15 ppm of CYA in a commercial swimming pool in the event of a fecal incident. It's simply impractical to try and kill crypto through hyperchlorination with elevated levels of CYA because the contact times are ridiculous. Check out the contact times in this screenshot from this CDC report:

Add CYA into that mix? It gets even harder to kill Crypto. Hence, the 15 ppm recommendation for CYA. Here is the PDF of the CDC's official Crypto incident response when CYA is in the pool.
Chasing pH is a losing strategy
Given this information, why are we constantly chasing a lower pH? Some say it's for bather comfort, or protecting pool surfaces and equipment.9 But the main justification is for chlorine strength (%HOCl). As you just saw in the graph above, that reasoning does not apply in stabilized pools.
It's hard to keep pH still because it is an equilibrium that fluctuates rapidly when pH adjustment chemicals are added to the pool. We also know that pH is largely dictated by the amount of carbon dioxide (CO2) in the water, thanks to carbonate alkalinity being our primary pH buffering system. So when CO2 leaves the water, the pH goes up. Hence, why aeration, like vanishing edges and spa spillovers, tends to raise pH faster than in a normal swimming pool without splash features.
So we'll ask our question another way. Do you think chasing pH is an effective strategy? And furthermore, do you think you can ever control it?
The pH ceiling and Henry's Law
The fact is, mankind cannot control pH, but we can leverage physics to contain it. In swimming pools, we know the pH can only naturally rise to a certain point, thanks to Henry's Law of Solubility of Gases.10 It states that any given gas dissolved in a liquid will eventually equalize with that same gas in the air above the liquid.
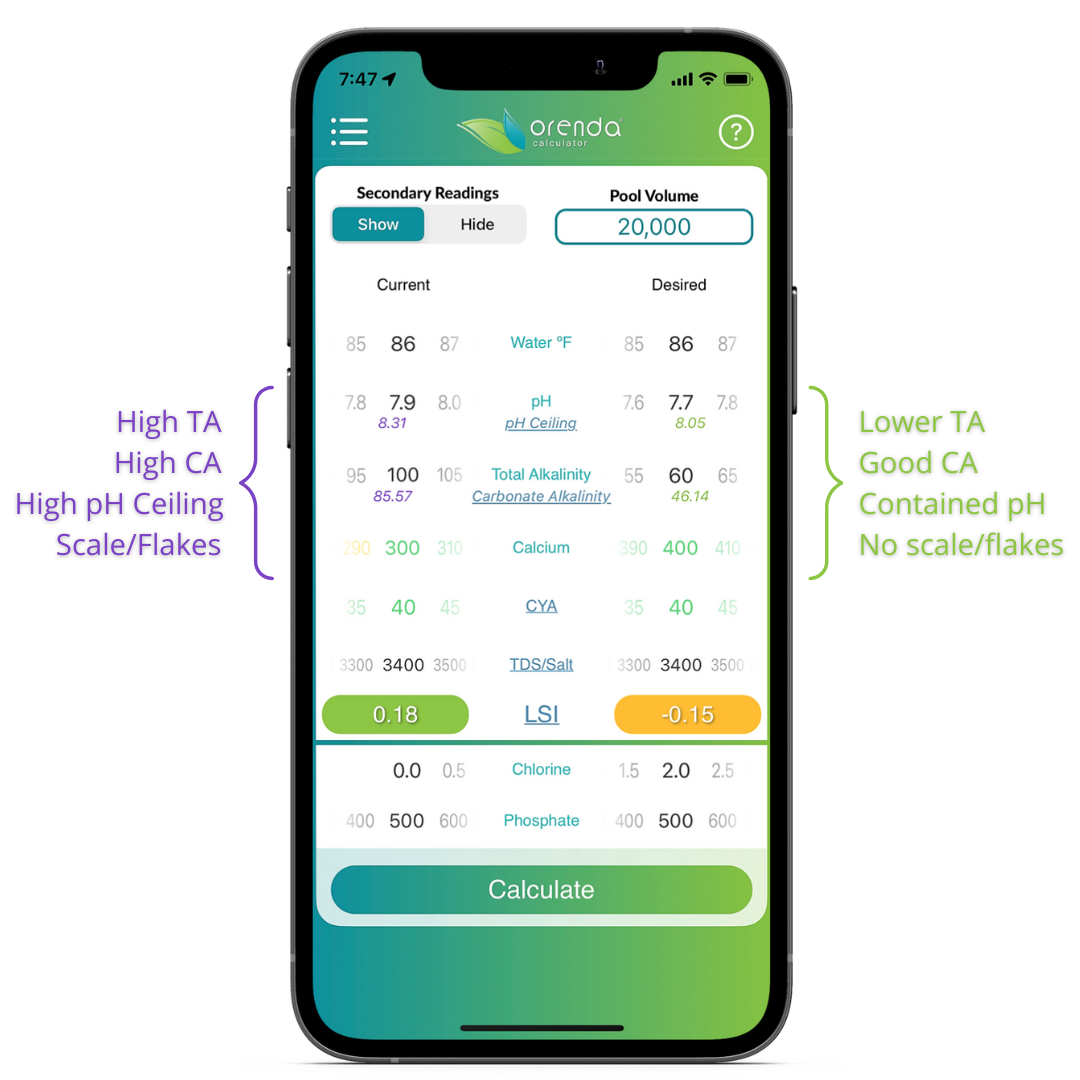
This means the CO2 in the pool will eventually equalize with the CO2 in the air above the pool. When equalized, the pH can no longer rise. We have coined this pH equilibrium point (pH(eq)) the pH Ceiling. We have added the pH ceiling in the Orenda Calculator™ as a secondary reading.

Swimming pools naturally rise in pH. It's just physics, and it will continue to happen. You are not doing anything wrong if your pH keeps climbing on you. It is important to know that if the pH goes above the pH ceiling, then it was forced unnaturally. Usually by etching plaster, or adding too much of a chemical like soda ash.
Related: CO2, pH and Henry's Law
Limit your pH ceiling to better protect chlorine from sunlight
Go back and look at the bottom right corner of the chart on the right. Look at the blue line, Hypochlorite Ion (OCl-). Do you see how as your pH gets higher, the blue line (OCl-) also rises? When the pH gets high enough, OCl- dissociates from CYA, and can be destroyed by sunlight.
So while pH has a negligible impact on %HOCl with CYA in your water, you need to be careful not to let your pH get up over 8.3 or so. Not only because 8.3 will lead to scale (bicarbonate converts into carbonate, increasing the likelihood of scale formation), but because chlorine will break away from CYA and be lost to the sun.
So instead of focusing on trying to maintain a virtually-impossible 7.2-7.6 pH, thinking it will keep your chlorine strong in a CYA pool, focus instead on your pH ceiling. Keep it below 8.3. The Orenda Calculator™ can help you with this, with the help of our secondary readings, which show you the pH ceiling in real-time.
Conclusion
When CYA is in the water, it impacts the concentration of HOCl (%HOCl) more than pH does. This is one of the main reasons why having minimal CYA is our fourth pillar of proactive pool care.
The free chlorine to cyanuric acid ratio (FC:CYA) is paramount for effective disinfection in swimming pools. It matters more than just free chlorine ppm! In short, cyanuric acid fundamentally changes the chlorination game.
Do not misunderstand what we are saying here. pH is still the most dominant of the six LSI factors, so it matters a lot for water balance. But we also know it is reactionary and should not be controlled. Instead, we recommend containing pH using its natural ceiling (based on physics and carbonate alkalinity), and a foundation of LSI balance as its floor. At least then the pH is predictable, and you're not wasting effort, time, and money trying to fight it.
It helps to have higher levels of calcium hardness and lower levels of alkalinity if drifting pH is an issue, as lower carbonate alkalinity can lower the natural ceiling for pH.
As a final note, this article is about outdoor chlorinated pools. pH still controls chlorine strength for pools without CYA, like indoor pools. Thanks for your time, and if you have more questions, please comment below.
1 North Carolina Area Wide Optimization Program Team. (2020). The CT Method: A Reference Guide. NC DEQ.
2 There is such an abundance of research on cyanuric acid's impact on chlorination, we stopped counting after finding over a dozen articles. We have cited several of them here in this article, though we encourage you to do further research if you want to find more. It's not hard to find online.
3 We at Orenda recommend not exceeding 15 ppm of cyanuric acid in public outdoor pools, per the CDC's recreational water guidelines for accidental fecal incident response. We recommend not exceeding 50 ppm of cyanuric acid in private residential pools.
4 Between a pH of 7.0 and 8.5, the difference in %HOCl is negligible. However, the pH matters above 8.0, and it matters more as the pH increases from there, due to OCl- dissociating from CYA and becoming vulnerable to sunlight decomposition (photolysis). Learn more in our other article about this.
5 Pickens, Stanley. (2017). Relative Effects of pH and Cyanurate on Disinfection. White Paper. Downloadable PDF.
6 The Council for the Model Aquatic Health Code (CMAHC) had an ad-hoc committee to study Cyanuric Acid. Their initial report is full of good information about it. You can read their presentation here.
7 Canelli, E. (1974). Chemical, Bacteriological, and Toxicological Properties of Cyanuric Acid and Chlorinated Isocyanurates as Applied to Swimming Pool Disinfection: A Review. American Journal of Public Health (AJPH), Vol. 64, No.2 (pg. 157).
8 J. O'Brien, J. Morris, J. Butler. (1974). Equilibria in Aqueous Solutions of Chlorinated Isocyanurate. A. Rubin, ed., Chemistry of Water Supply, Treatment and Distribution. Ch. 14, pp. 333-358.
9 The idea of pH needing to be 7.2 to 7.8 for "bather comfort" (because of the pH of our eyes) is a myth. There is a study published on the official NIH website that found that eye irritation is usually from osmotic pressure due to the salinity difference between eyes and pool water. Slightly more alkaline water is unlikely to irritate our eyes. Eye irritation is caused more by disinfection byproducts (DBPs) like chloramines and trihalomethanes (THMs) like chloroform than pH. Related: What is the ideal pH for swimming pools?
10 Khan, Sannah. (Retrieved 2024). Henry's Law. LibreTexts, Chemistry. Shared under a CC BY-NC-SA 4.0 license by the California Department of Education.
