Understanding Calcium Hardness

Calcium hardness is one of the most important factors in water chemistry, and swimming pool chemistry especially. It is one of the six LSI factors, and because it does not fluctuate much, we love using calcium hardness as a foundation for water balance. Let's discuss why.
Covered in this article:
- What is calcium hardness (CH)?
- Elemental calcium (Ca)
- Calcium ion (Ca2+)
- Calcium carbonate (CaCO3)
- Why is calcium hardness expressed as CaCO3 when it's technically a measure of calcium ions?
- Calcium's Role in the Langelier Saturation Index (LSI)
-
- What is the ideal calcium hardness level in a swimming pool?
- Common types of calcium carbonate issues in pools
- Calcium carbonate scale
- Carbonate clouding and dust
- Calcium nodules
- Calcite crystals
- Uneven carbonation
-
- What raises and lowers calcium hardness?
- Testing for calcium hardness in tap water
- Conclusion
What is Calcium Hardness (CH)?
Calcium Hardness (CH) is how much calcium is dissolved in water, measured in parts-per-million (ppm, or mg/L) of calcium carbonate (CaCO3). Technically, calcium hardness is measuring calcium ions, which are usually bound to anions to form compounds like calcium carbonate, calcium sulfate, or calcium chloride. Calcium carbonate, as we will mention in a moment, is the bedrock of water balance. It is the foundation to build a solid water chemistry strategy upon.
Total Hardness (TH) refers to the level of calcium hardness along with other minerals like magnesium. Test kits in the swimming pool business usually just test for calcium hardness. Calcium hardness is the factor we need to calculate the Langelier Saturation Index (LSI).
Elemental Calcium (Ca)

Calcium, in its elemental form, is an alkali earth metal (atomic number 20) on the Periodic Table of Elements.1 Like other alkali earth metals in this Periodic group of elements, Calcium has two valence electrons in its outer ring, which are quickly lost.
When elemental Calcium loses its electrons, it becomes the calcium cation, meaning it's an ion with a positive charge. The positive charge comes from the fact that the two valence electrons left the atom, meaning its valence becomes net-positive by two protons. To reduce confusion, we'll just call it calcium ion (Ca2+).
Because of this, calcium and other alkali earth metals–like magnesium–are not found in their pure elemental form in nature. They are always bound to other elements.2
Calcium ion (Ca2+)
Once the valence electrons have left elemental Calcium, the calcium ion is positively charged with two protons (Ca2+). To stabilize itself, the calcium cation seeks a negatively-charged anion. In swimming pools, there can be some anions like Sulfate (SO42-), Phosphate (PO43-), and Silicate (SiO44-) that can bind to calcium.3 But these are rare compared to the dominant ions of alkalinity.
Bicarbonate (HCO3-) and Carbonate (CO32-) alkalinity anions bind quickly with calcium to form calcium bicarbonate (Ca(HCO3)2) and calcium carbonate (CaCO3).
Calcium bicarbonate is soluble and as the pH rises, it quickly converts to calcium carbonate:
Ca(HCO3)2 → CaCO3↓ + CO2↑ + H2O
Calcium bicarbonate → Calcium carbonate (dissolved) + carbon dioxide (off-gas) + water
And while calcium bicarbonate exists in water, calcium hardness is measured in ppm of calcium carbonate.
Calcium Carbonate (CaCO3)
Get used to hearing us talk about this substance. If you know nothing about water chemistry, know that dissolved calcium carbonate is very important to water. As mentioned earlier, the amount of calcium ions is calcium hardness, expressed as ppm (or mg/L) of CaCO3. The reason for this is because of the molecular weight of calcium carbonate.
Why is calcium hardness is expressed as CaCO3 when it's technically a measure of calcium ions?
CaCO3 is 100 g/mol, and water is 1000 g/Liter. This makes for easy math when calculating mg/L, which is the metric equivalent to parts-per-million (ppm). That's why calcium hardness is expressed as CaCO3 instead of just calcium ions.
Calcium carbonate (CaCO3) is created when calcium ion is carbonated. This can occur a few different ways. For example, when calcium hydroxide in cement is converted by carbon dioxide:
Ca(OH)2 + CO2 → CaCO3 + H2O
Calcium hydroxide + carbon dioxide → calcium carbonate + water
When cementitious pool surfaces are curing underwater (during the initial 30 days of filling the pool, called startup), the goal is to carbonate the soluble calcium hydroxide in the cement into less-soluble calcium carbonate. Either that's done by CO2 in the water or from carbonate ions in the water.
Carbonation can also occur when carbonate ions in the water bind to calcium ions in the water:
Ca2+ + CO32- → CaCO3
Calcium ion + carbonate ion → Calcium carbonate
We could keep talking about calcium carbonate and all of the things it affects in pool chemistry, but we want to respect your time. If you want to learn more, we encourage you to research it further. The main takeaway is the amount of CaCO3 (ppm or mg/L) is calcium hardness, but how saturated the water is with that calcium hardness is the LSI.
Calcium's role in the Langelier Saturation Index (LSI)
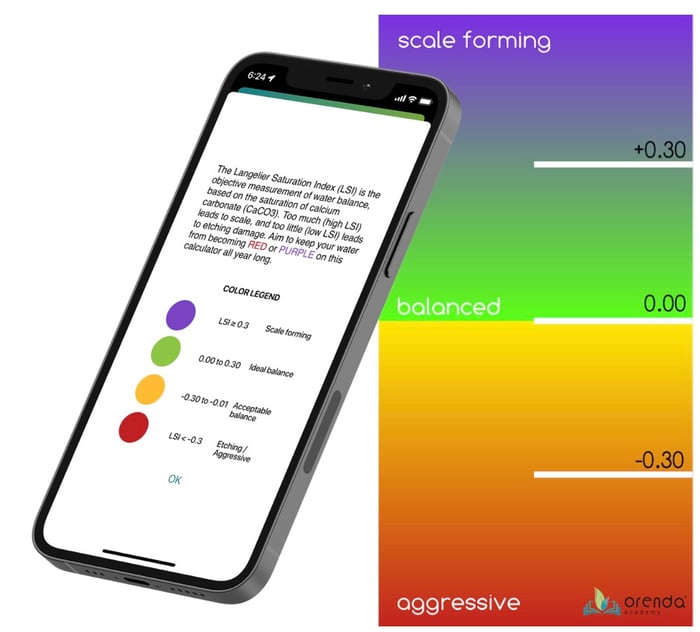
Water Balance is defined by the saturation equilibrium of calcium carbonate. The international standard for measuring this balance is the Langelier Saturation Index (LSI), which is the focus of the first of our Four Pillars. Calcium Hardness is the bedrock of the LSI.
Calcium hardness is a factor that does not change much unless you add calcium or dilute the water to reduce it. It's the baseline amount of calcium carbonate dissolved in water, whereas the other LSI factors determine how saturated the water is with that calcium carbonate.
The LSI is calculated using a formula that involves six (6) factors.4 They are:
- Water temperature
- pH
- Carbonate Alkalinity
- Calcium Hardness (CH)
- Total Dissolved Solids (TDS)
- Cyanuric Acid (CYA)
The LSI formula is done automatically in the Orenda Calculator™ for you. If you want to dig deeper, you can read our article about Understanding LSI to see the actual LSI calculation formula.
The LSI tells us how saturated the water is with calcium carbonate in the present conditions (meaning at the exact values for each of the six LSI factors). So by changing any of those six factors, we can change the condition of the water, which changes the solubility of calcium carbonate. Perfect saturation is 0.00 LSI. An LSI above +0.30 means the water is oversaturated with calcium carbonate. An LSI below -0.30 means the water is undersaturated and wants more calcium carbonate.
While we have six factors to play with, and therefore countless options to balance water. But water has only two ways to balance itself: eat or scale. If the LSI is too low, water will have to eat (etch cement and dissolve calcium into solution). If the LSI is too high, water will have to scale (precipitate CaCO3 to get back to balance).
What is the ideal calcium hardness level in a swimming pool?
The ideal calcium hardness level for your swimming pool is whatever allows you to maintain LSI balance year-round. This depends on your climate, temperature swings, annual rainfall, primary sanitizer type, and other factors. Generally speaking, the colder your winter, the more calcium hardness your water needs.
Common types of calcium carbonate issues in pools
Without diving too deep into each of these issues, we just want to mention the most common types of calcium problems in swimming pools. These issues are all related to the LSI, and they are all forms of calcium carbonate. Listen to the podcast episode for more information on the issue.
Calcium carbonate scale
Scale is a hardened calcium deposit that precipitated from water (due to a high LSI violation) and landed on a pool surface or equipment. The most common form of scale–by far–is calcium carbonate scale. But there are other calcium compounds that can form scale that are not based on the LSI, like calcium sulfate scale, calcium phosphate scale, and calcium silicate scale. Thankfully these others are more rare, as they are far more difficult to clean up and remove.
Calcium carbonate scale can be prevented by simply balancing the LSI, and if necessary, chelating calcium in the water with SC-1000.
Scale will always present itself in the highest-LSI places first. This is usually the heater and/or salt cell (if you have one), or the tile line. This is because these areas are the warmest water in the pool, and warmer water temperature raises the LSI. You will not see scale on the bottom of the pool if it's not everywhere else.

Carbonate clouding and dust
If you have ever turned the heat on in a spa and it clouded up, or added soda ash to a pool and it clouded up, then you have witnessed a high LSI violation in real time. Carbonate clouding is when CaCO3 precipitates due to a high LSI in that moment. It can happen when adding cal hypo chlorine, soda ash, sodium bicarb, or simply turning up the heat. It happens when the LSI is raised too high, and rapidly.
Eventually this cloudiness either redissolves, or it falls to the floor as calcium carbonate dust.
Calcium nodules and efflorescence
Unlike the other four calcium issues mentioned here, evidence suggests that efflorescence and calcium nodules are not caused by an LSI violation. Rather, calcium nodules are the result of moisture trapped in or behind plaster material, which is then forced out toward the pool. Efflorescence is similar, in that mineral salts are pushed out from within cement due to moisture pushing through it, which then deposits calcium carbonate on the other side.
Calcium nodules are related to the LSI, however, because once the calcium hydroxide is forced out from within the cement, it interacts with alkalinity in the water, and carbonates into calcium carbonate to form a nodule.
Calcite crystals
Calcite crystals are the opposite of calcium carbonate scale, in that they grow from within the cement. Scale originates from the water and lands on surfaces, whereas these crystals are drawn out from cement into the water. Much like a calcium nodule (except that aggressive, low-LSI water causes this), the crystals form as calcium hydroxide comes out of the cement and gets carbonated.
Uneven carbonation
Of all the calcium issues that people call Orenda about, uneven carbonation is probably the most common. These issues might include "ghosting" or "graying" of plaster surfaces, mottling, and other discolorations on cementitious surfaces. The defining feature of uneven carbonation is caused by aggressive water pulling pigment out of the surface, drawing calcium hydroxide to the surface, which then carbonates pure white. The way you can tell is if the white marks are around aggregates (like pebbles) but not on top of them. Like this:

What raises and lowers calcium hardness?
We have another article about what raises and lowers specific chemistries in water, but we'll include it here too.
In short, if calcium levels increase, the calcium had to come from somewhere. Either
- you add it (via calcium chloride or cal hypo chlorine),
- it feeds into the pool from tap water, or
- the water is dissolving it from cement somewhere due to a low LSI violation.
If calcium levels decrease, the calcium has gone somewhere. Either
- it has come out of solution (due to a high LSI violation that precipitated calcium carbonate in the form of scale, carbonate clouding, or dust) and can therefore no longer be measured by a test kit, or
- whole water was lost or removed (via backwashing, splash out, dilution, or a pool leak), or
- the pool was filtered with reverse osmosis (RO), which removed calcium.
Testing for calcium hardness in tap water
All water chemistry starts at the tap. Testing tap water is essential to proactive pool care and LSI-based startups. As we just mentioned in the last section, calcium hardness does not go away unless you remove whole water from the pool or filter it out. Calcium hardness accumulates, especially in pools in dry climates with high evaporation rates.
Take our word for it, you want to know the calcium level (and everything else) in your source water. Test it annually and write it down so you don't forget. A post-it note inside the test kit case is not a bad idea.
Conclusion
Calcium is a reliable foundation for LSI balance, and, therefore a solid foundation for your entire water chemistry strategy.
Unlike other factors like pH and total alkalinity—which fluctuate quickly and often—calcium hardness is remarkably stable. While the other factors can be moved around to change the conditions of the water, calcium carbonate itself is what water craves. Play around with the Orenda Calculator™ and move calcium up and down to see its impact on the LSI. You will quickly see that elevated levels of calcium hardness allow you to have lower alkalinity; which in turn creates a lower pH ceiling; which in turn allows you to contain pH and predict where the LSI will be in a week.
Without enough calcium hardness, water balance is virtually impossible.
1 PubChem element summary for Calcium. https://pubchem.ncbi.nlm.nih.gov/element/Calcium
2 While there is an abundance of credible information about calcium online, we found the best explanations are the simplest. This source for middle school education explains elemental calcium and other alkali earth metals cogently: Flexbooks 2.0. (Updated 2019). 4.8 Alkaline Earth Metals. CK-12 Physical Science for Middle School.
3 These anions bind with calcium to create calcium sulfate, calcium phosphate, and calcium silicate. All three of these compounds can precipitate out of solution from the water and form different types of scale. Because they are not calcium carbonate, the LSI has nothing to do with it.
4 Dr. Wilfred Langelier's original saturation index had only five factors: water temperature, pH, carbonate alkalinity, calcium hardness, and total dissolved solids (TDS). It was originally created for closed-water systems, like boilers. The index was adapted to other water systems, including swimming pools. In swimming pools, other chemistries can contribute to total alkalinity that must be accounted for and removed, because the LSI needs the carbonate alkalinity only. These factors include cyanurate alkalinity, and if you use them, borates. So we say there are six (6) primary factors of the LSI, which includes cyanuric acid (CYA). The optional seventh factor would be borates, but only if you use them. We have the option in the Orenda App > menu > settings to include borates in the Orenda Calculator™. When you have CYA and/or borate in the water, our calculator will automatically correct against the Total Alkalinity and display your carbonate alkalinity as a secondary reading.