Total Alkalinity vs. pH, and their roles in water chemistry

Have you ever asked "what's the difference between pH and alkalinity? Many of us in aquatics confuse total alkalinity and pH. It’s understandable, given how blurred the line is between words like “alkaline” and “alkalinity.” Indeed, alkalinity and pH in water chemistry are closely related, but they are not the same. This article will distinguish between them.
If reading is not your thing, you can listen to our podcast! Our very first episode is about pH and total alkalinity:
Our podcast is available in most mobile audio platforms such as spotify, apple podcasts, amazon music, etc, and also in our website.
Covered in this article:
- What is pH?
- The roles of pH in water chemistry
- Dissolved CO2 determines pH
- What is Total Alkalinity (TA)?
- The role of TA in water chemistry
- How to raise TA and pH
- How to lower TA and pH
- Conclusion
What is pH?
We have our own videos and podcasts explaining pH, but here's a phenomenal explanation of it from a medical perspective. After all, blood is mostly water, and we're talking water chemistry. The same rules apply in swimming pools:
pH stands for potenz Hydrogen, more commonly referred to as the "power of Hydrogen". It is the negative logarithm of Hydrogen ion concentration in a solution. The lower the pH, the exponentially more Hydrogen.
pH = -log[H+]
We measure how acidic or basic (also called alkaline) substances are by using the pH scale. The pH scale goes from 0 to 14, and is relative to pure water, which has a perfectly neutral pH of 7. When the concentration of hydrogen ions goes up, the pH goes down (and vice versa).
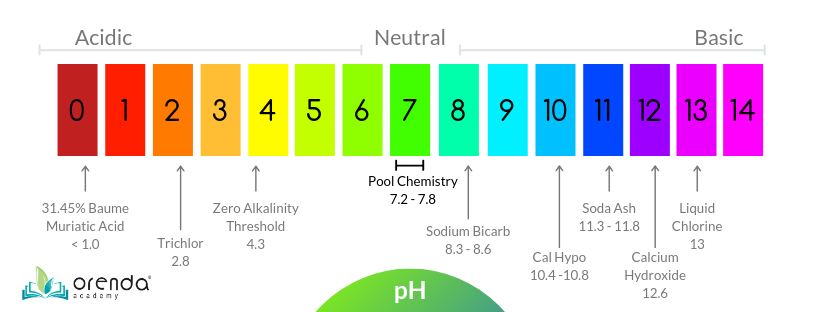 The scale is logarithmic, which means each whole number on the 1-14 pH scale is 10 times more or less than the numbers around it. For example, something with a pH of 6.0 would be 10 times more acidic than 7.0 pH distilled water. Something with a pH of 5.0 is 10x more acidic than 6.0, and 100x more acidic than 7.0 (10x10=100).
The scale is logarithmic, which means each whole number on the 1-14 pH scale is 10 times more or less than the numbers around it. For example, something with a pH of 6.0 would be 10 times more acidic than 7.0 pH distilled water. Something with a pH of 5.0 is 10x more acidic than 6.0, and 100x more acidic than 7.0 (10x10=100).
To put the pH scale in perspective, let’s do some quick math. Trichlor, a popular type of stabilized chlorine used primarily in outdoor pools, has a pH of about 3. For this math, let’s just round it to exactly 3.0. How acidic is 3.0 pH Trichlor compared to 7.0pH water? Let’s figure it out.
(7.0 pH - 3.0 pH) = 4.0
pH is logarithmic, so that means 104, or 10x10x10x10 = 10,000
Trichlor is about 10,000 times more acidic than pure water.
This math is just to explain the pH scale itself and illustrate what logarithmic means.
Water (H2O) splits
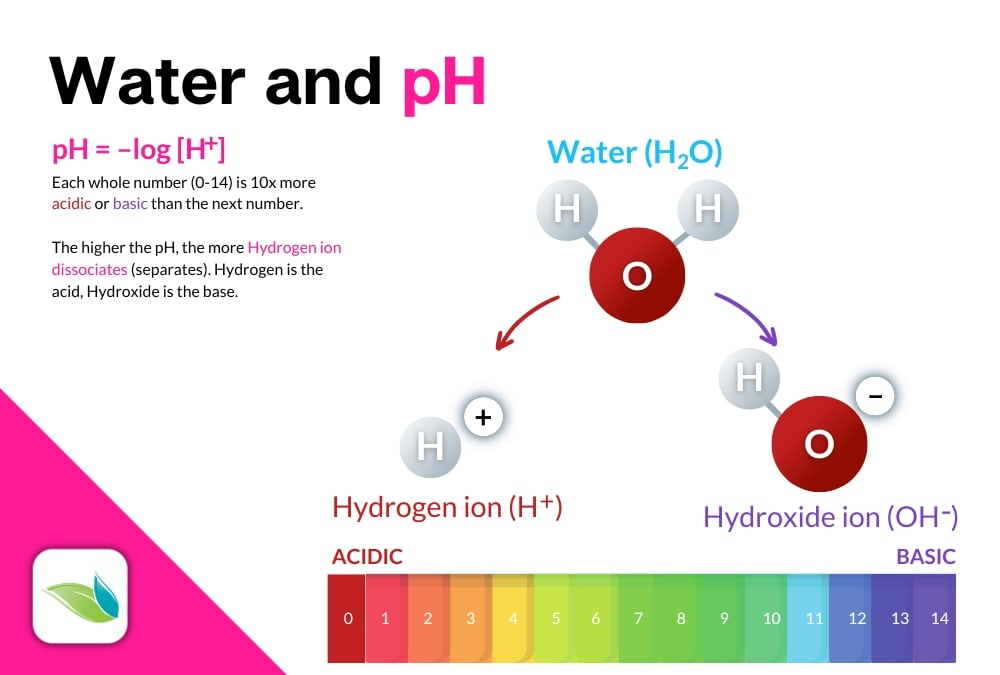
Another way to visualize pH is an H2O molecule of water. When a Hydrogen ion (H+) dissociates or breaks away, it leaves behind a Hydroxide ion (OH-). The H+ is the acid, and the OH- is the base. So when you see substances that have an (OH) in them, such as sodium hydroxide (NaOH), or calcium hydroxide (Ca(OH)2), you know it will have a high pH.
The roles of pH in water chemistry
The industry standard pH range is between 7.2 and 7.8, according to PHTA standards. The ideal pH, we are told, is 7.4-7.6. But why? Three main reasons are cited: chlorine strength, bather comfort, and corrosivity/scale formation in water. Let's take a moment to debunk all three...or at least two and a half.
Chlorine strength (%HOCl)
Health departments care mostly about water being maintained with adequate disinfection speed. So of the three reasons cited for maintaining a pH between 7.2 and 7.8, by far the most important to the health department is chlorine strength (%HOCl).
Indeed, pH determines how effective chlorine will be in non-stabilized pools. So in pools with no cyanuric acid (CYA), the lower the pH, the stronger chlorine will be, because pH controls the dissociation equilibria between strong and weak chlorine:
HOCl ⇌ H+ + OCl-
Hypochlorous Acid dissociates into Hydrogen and Hypochlorite Ion
Because pH is about the concentration of Hydrogen ions (H+), the lower the pH, the higher the percentage of HOCl vs. its far-weaker and slower counterpart, OCl-.
But this is not as relevant in pools with CYA, because isocyanurates fundamentally change pool chemistry. See the graphic below. The chart on the left shows a non-stabilized pool (zero CYA), compared to a stabilized pool (30 ppm CYA):
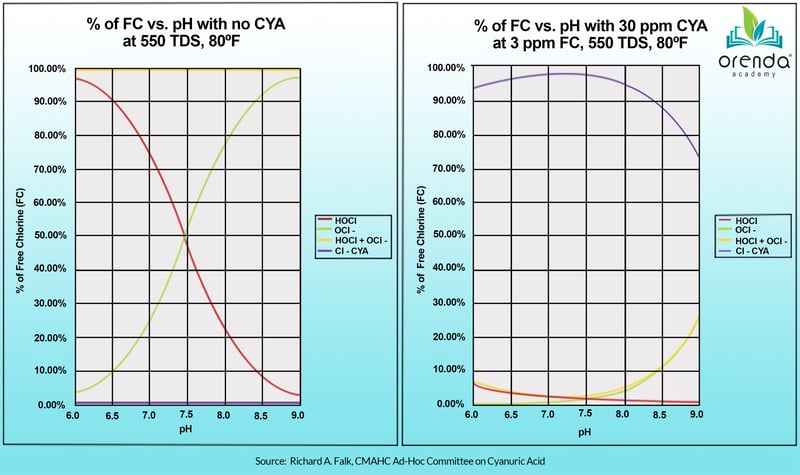 Where's the red line now?
Where's the red line now?
Do not let this chart mislead you. With CYA in your pool, you will still have chlorine, it's just not as pH-dependent because most if it is bound to CYA. More on pH, CYA and chlorine relationships here.
So this notion of pH controlling chlorine strength is at least half true. The other half is that pH is not driving the strength of chlorine in pools with CYA in them (which is virtually every outdoor pool in America).
Bather comfort (myth)
The second main reason the textbooks say we need to maintain a pH between 7.2 and 7.8 is due to bather comfort. Supposedly the pH of our eyes matters enough that the pH of the water has to be compatible with them, or else swimmers' eyes will hurt. But we believe this is a myth, and we can fairly easily debunk it.
- First, the pH of your eyes (or tears, specifically) depends on how hydrated you are. One published study showed the pH of human tears in 44 test subjects ranged from 6.5 to 7.6.1
- Second, in over-a-decade of competitive swimming experience, we believe it's actually chloramine byproducts that are causing bather irritation. It's no secret that chloramines and other disinfection byproducts are noxious and irritating to eyes, skin, throat, and lungs. The combined chlorine issue is thoroughly studied and well-understood.
- Third, the pH of bottled drinking water ranges far more than a swimming pool. Common brands range from 5.5 pH to over 9.5 pH. That's a 10,000x difference in acidity! And yet we can hardly taste the difference. Both are refreshing and able to be consumed. Are we really to believe that a swimming pool, kept WAY within that pH range has enough pH difference to irritate bathers skin and eyes? We don't buy it.
Related: What is the ideal pH in a swimming pool?
Etching/Scaling (actually the LSI)
The third reason pH is supposed to be kept between 7.2 and 7.8, we are told, is due to corrosivity orscale-forming tendencies of water. In other words, low pH destroys pool surfaces and corrodes equipment (like heaters), whereas high pH leads to carbonate scale.
Fair enough...except that such things are actually dictated by the total balance of water, not just pH. We measure the total balance of water using the Langelier Saturation Index (LSI). The LSI determines when water is oversaturated or undersaturated with calcium carbonate (CaCO3). And pH is a major factor in the LSI.
LSI violations occur locally, not universally throughout a pool. Therefore pH matters a lot. For instance, 2.8 pH Trichlor in a floater can cause damage if it's floating over a shallow step, or next to a wall for extended periods of time. The low pH changes the LSI in that immediate area, causing that area to become corrosive and etch the cement surface (or fade the vinyl liner, or degrade the fiberglass gel coat).
There's a chart below that shows the equilibria of alkalinity types. When the pH gets to 8.3 and above, bicarbonate ions convert into carbonate ions (CO32-), and they bind really well with calcium (Ca2+). So when your pH gets too high (over 8.3), calcium can precipitate out of solution even if the overall LSI of your pool is balanced. You can see this phenomenon for yourself. Just add soda ash too quickly and watch it immediately cloud up the pool.
So pH alone is not the reason for etching or scale formation–the LSI is. But pH is a major factor in the LSI. Low pH can be compensated for with more calcium hardness, alkalinity, and higher water temperature, however.
Dissolved CO2 determines pH
Technically the pH is the concentration of Hydrogen ions, expressed logarithmically between 0-14. But that's complex and hard to wrap our heads around. So for practical purposes, a better way to conceptualize pH is by thinking about the amount of carbon dioxide (CO2) dissolved in your water.
The more dissolved CO2 in your water, the lower the pH, and vice versa. This should explain why CO2 injectors lower pH, and why aeration (such as splash features, spillovers, and spa jets) raises pH. We cover this much more in-depth in other articles:
Now that we have covered pH, let's move on to alkalinity.
What is Total Alkalinity?
Total alkalinity is a measurement of the concentration of all alkaline substances dissolved in the water that can both attract and release Hydrogen ions (H+). This interference with Hydrogen is why alkalinity buffers against change in pH. Total alkalinity is primarily bicarbonate, carbonate, and hydroxide, along with a few others like cyanurate alkalinity. When acid is added, these alkali have the ability to neutralize some of the acid. In simpler terms, total alkalinity measures the water’s ability to resist a reduction in pH.
Related: What is Alkalinity?
Total alkalinity is measured by its concentration in parts-per-million (ppm), and the industry-standard ideal range is from 80-120 ppm, depending on the type of chlorine you use. For example, Trichlor has a low pH of 2.8, so given how acidic Trichlor is, a trichlor pool needs higher alkalinity. Another reason for more alkalinity in a trichlor pool is because the CYA in trichlor offsets alkalinity when doing LSI corrections.
Non-stabilized chlorines, like liquid chlorine (bleach) or cal hypo, each have a high pH. So you can have lower alkalinity, like 80 ppm, or even lower than 80 ppm if your LSI allows for it. Salt chlorine pools will often struggle with scale buildup that flakes off the salt cell and blows into the pool. Salt pools, in season, can usually benefit from lower levels of alkalinity, like 60 ppm, provided the LSI remains balanced. The key is to measure acid and dilute it before pouring to avoid overcorrections. Too much acid with lower alkalinity will cause bigger problems, so be careful.
And yes, we are aware we are recommending lower total alkalinity levels than the textbook standards (provided your LSI remains balanced, so keep water temperature in consideration!). The lower alkalinity will allow you to contain pH more effectively.
Related: Understanding pH buffering systems and pKa values
How alkalinity is measured
Did you notice that total alkalinity is not measured on the pH scale? This is an important distinction to make because this is usually how pH and alkalinity get confused. Total alkalinity is measured by the amount, or concentration (ppm) in the water, not by how alkaline the water is.
Alkalinity cannot be measured in real-time with probes, at least with today's technology. Perhaps someday it will be possible, but for now, we must rely on titration or color-change on a test strip. The most common titration is with liquid reagents, where each drop is counted as it neutralizes more and more of the sample until the color changes. Follow your test kit manufacturer's instructions.
The reason this is worth mentioning is that chemical automation controllers can read pH in real-time with a probe, but controllers do not know what the alkalinity of the water is. Depending on the controller, this can lead to overuse of acid, which can compound into much bigger issues.
The role of Total Alkalinity in water chemistry
If you’re a pool operator, you probably already know that pH fluctuates. In particular, it naturally rises. Correcting pH is almost always accomplished by lowering it with acid or injecting CO2.
Chasing pH means you are fighting against physics, and the water is more difficult to maintain. Enter alkalinity. A simple way to think about alkalinity is that it insulates pH from being reduced too easily. In other words, alkalinity buffers against a reduction in pH. Alkalinity ions do this by either releasing or attracting a Hydrogen ion (H+) as needed. So when acid is added, carbonate ions can absorb Hydrogen to create bicarbonate ions. And with enough acid, bicarbonate ions absorb another Hydrogen and convert into carbonic acid, which is dissolved CO2. This is all in equilibrium, just like pH, so it can go the other way too. The equilibrium looks like this:
CO2 + H2O ⇌ H2CO3 ⇌ HCO3- + H+ ⇌ CO32- + 2H+
carbon dioxide + water ⇌ carbonic acid ⇌ bicarbonate + Hydrogen ion ⇌ carbonate + 2 hydrogen ions
Here's what the equilibrium looks like on a graph. As you can see, the pH determines the type of alkalinity you have, and most of our alkalinity in pool chemistry ranges will be bicarbonate:
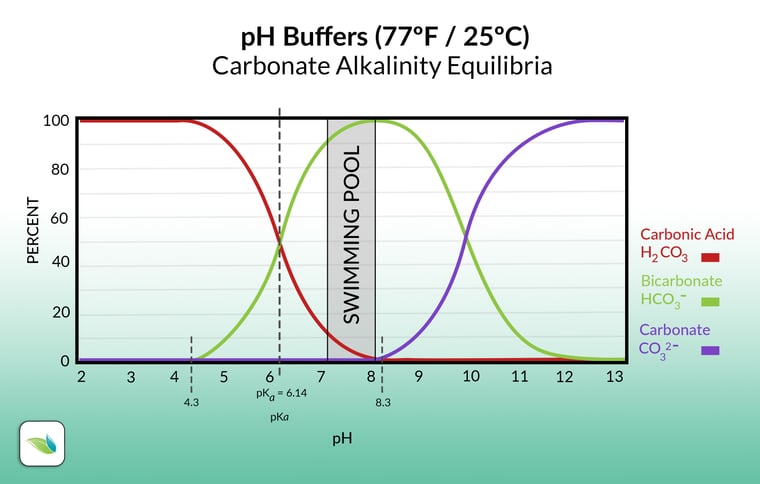
Total Alkalinity and pH reduction
Since CO2 determines the pH of the water, let's think about this another way. If you want to reduce pH, you need more dissolved CO2 (carbonic acid). You can either inject CO2 directly to lower pH, or you can use acid. But wait, acid doesn't contain CO2...so where does acid find CO2?
Acid converts alkalinity into dissolved CO2 (carbonic acid), which lowers the pH of the water.
Specifically, acid converts bicarbonate alkalinity into carbonic acid (H2CO3) by adding Hydrogen to it. Simplified, we think of it as acid 'burns through' alkalinity to reduce pH.
More Total Alkalinity leads to a higher pH
Contrary to most teachings, Total Alkalinity really only buffers against the reduction in pH. In fact, the more alkalinity in your water, the higher your pH will naturally rise...and generally speaking, the faster the pH will rise too. Here's the pH ceiling chart:
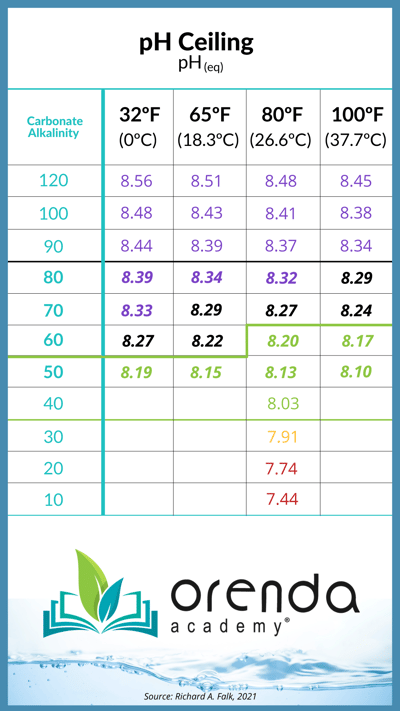
For more information on what this chart means, read this.
How to increase Total Alkalinity and pH
If you want to increase total alkalinity in a swimming pool, you need to know the gallonage of the pool and your pH. It helps to have an accurate dosing calculator. Raising alkalinity is as simple as adding sodium bicarbonate to the water.
You could add several pounds of 8.3 pH Sodium Bicarb and make the same pH impact if you added a much smaller amount of 11.6 pH soda ash. But the total alkalinity would be higher for the Sodium Bicarb because there’s simply more of it in the water.
Related: How to raise alkalinity and pH
How to lower Total Alkalinity and pH
You can lower pH and alkalinity using acid. Always dilute acid in a bucket of water, and evenly pour it around the deepest parts of the pool. Do not column pour acid, contrary to some industry practices. Column pouring offers no benefits compared to diluting acid. Column pouring can severely lower the pH in a concentrated location (at the bottom of your pool, which gets pulled directly into the main drain). This tanks the pH, and leads to equipment damage. Always dilute acid.
Related: How to lower pH and Alkalinity
Conclusion
pH tells us how acidic or basic a substance is, on a 0-14 logarithmic scale, telling us the concentration of Hydrogen ions (H+). Total Alkalinity (TA) is the amount of dissolved alkali in water that can both give and take Hydrogen ions. Because of this interference with Hydrogen, alkalinity serves as a buffer against the reduction in pH.
Both pH and TA are important in water chemistry. Both are factors in the overall balance of water (measured using the LSI). Total Alkalinity includes carbonate alkalinity and other forms too–primarily cyanurate alkalinity. The carbonate alkalinity determines how high the pH of the water can rise (pH ceiling), thanks to Henry's Law.
So when you're maintaining your water, keep in mind that higher TA will mean more acid required to reduce pH, and it will also encourage pH to rise higher. Lower TA will mean less acid required to reduce pH, and will limit how high pH can go. We definitely want TA in our water, but we do not need as much as the textbooks say in some cases...it depends on the time of year (water temperature in particular), and the type of primary chlorine. Acid chlorine like trichlor needs more alkalinity, whereas salt chlorine systems, liquid chlorine and cal hypo can be fine with less than 80 ppm TA, provided you maintain LSI balance. We elaborate on this thoroughly in other articles listed above.
We hope this helps!
1 Abelson, M. B., Udell, I. J., & Weston, J. H. (1981). Normal human tear pH by direct measurement. Archives of ophthalmology (Chicago, Ill. : 1960), 99(2), 301. https://doi.org/10.1001/archopht.1981.03930010303017

