Understanding pKa Values and pH Buffering Systems in Water Chemistry
In this article, we explain how alkalinity buffers pH. We focus specifically on the significance of pKa values in water chemistry and how they impact the buffering capacity against changes in pH.
Covered in this article:
- What does pH buffering capacity mean?
- pH and hydrolysis
- How alkalinity buffers pH
- Acids and conjugate bases
- Defining pKa values and their importance
- Equilibrium between an acid and its conjugate base
- Conclusion
What does pH buffering capacity mean?
Let's start with the concept of pH buffering capacity, which refers to water's ability to resist changes in pH when an acid or a base is added.
In swimming pools, almost every chemical we use impacts pH in some way. In addition, pH impacts just about every other aspect of water quality and balance. Needless to say, pH is important, yet it easily moves. A pH buffer helps to slow the change in pH.
In swimming pools, total alkalinity (TA) measures all dissolved alkali in the water that can neutralize acids. In other words, TA measures the pH buffering capacity of our water. When acids are added, they introduce excess Hydrogen ions (H+), which combine with alkaline ions like bicarbonate (HCO3-) and carbonate (CO32-).
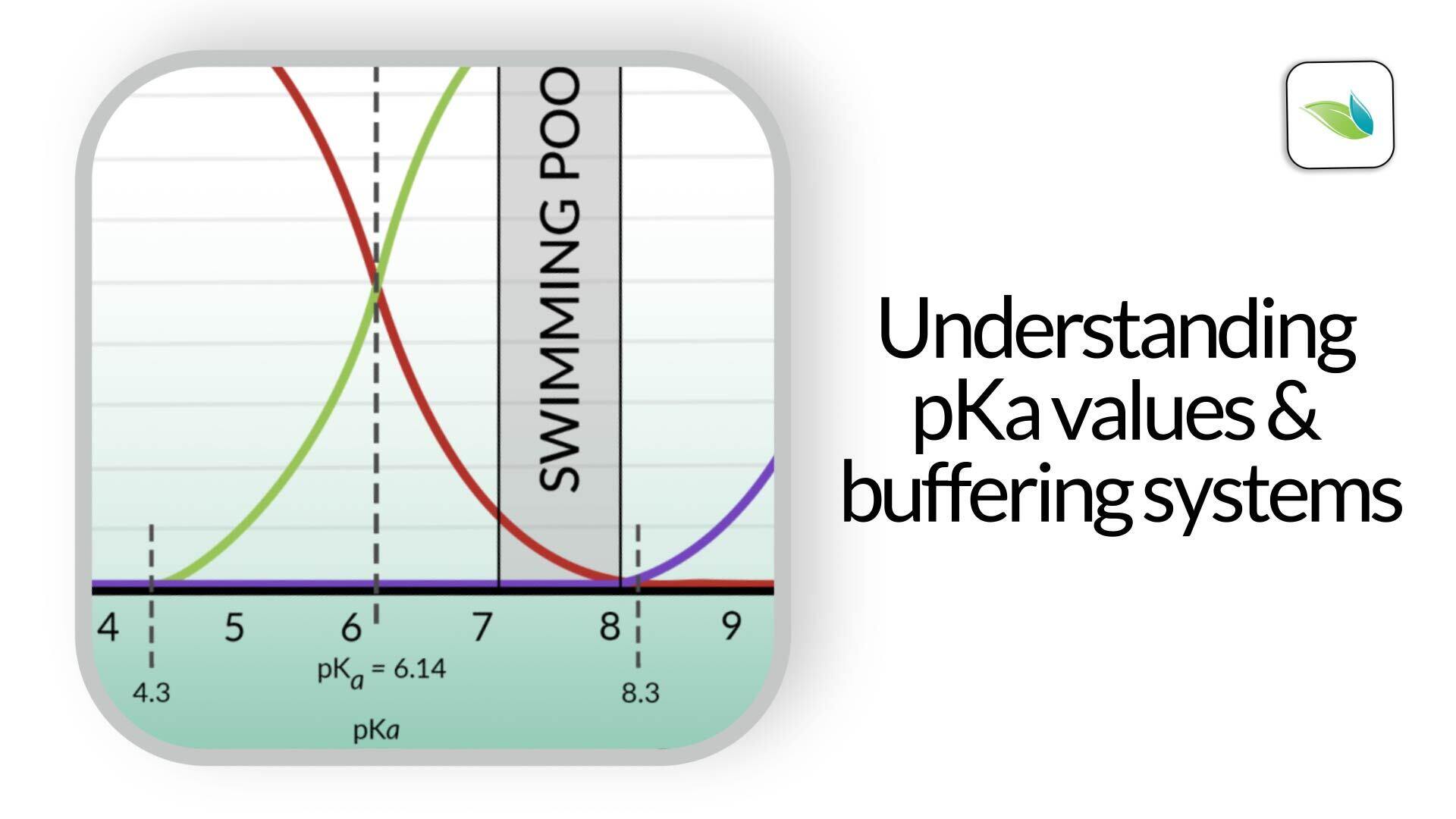
As you can see from the chart below, if Hydrogen is added to bicarbonate (HCO3-), it becomes carbonic acid (H2CO3), which lowers the pH of the water.1

pH and hydrolysis
We have several other articles about pH, including this explainer in our help center. In a quick summary, pH is the concentration of Hydrogen ions in a solution that measures how acidic or basic that solution is. It's based on how water (H2O) dissolves various substances in a process called hydrolysis.
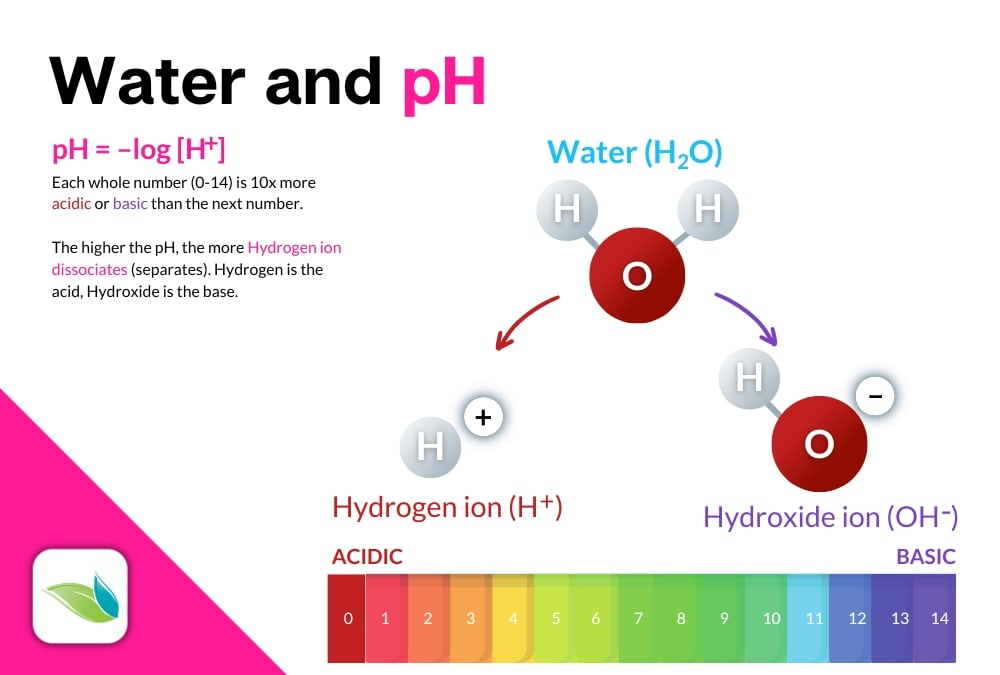
Hydrolysis is the technical term for 'dissolving.' It is a chemical reaction in which a compound is split while incorporating a water molecule (or water's components). For example, a common hydrolysis reaction is called ionic compound dissociation, like when salt dissolves in water:
NaCl → Na+(aq) + Cl-(aq)
Salt → Sodium(aqueous) + Chloride(aqueous)
Salt breaks apart into aqueous forms of sodium and chloride ions. But salt (sodium chloride, NaCl) does not change the pH of the water it dissolves into. Most pool chemicals do change the pH of the water, even if only in a minimal way.
Dissociation in water is important because it directly influences how buffering systems work.
How alkalinity buffers pH
Alkalinity ions stabilize the pH level because they can both take and give away a Hydrogen ion (H+). Because of this, alkalinity neutralizes acids, slowing the reduction in pH. An acid's Hydrogen ions will bind to the alkalinity ions in the water.
Water with high alkalinity (high buffering capacity) can neutralize more acids, meaning it has more resistance to a change in pH. Conversely, water with low buffering capacity (low TA) is more susceptible to large pH changes.2
Buffering systems like carbonate alkalinity and borate are composed of weak acids and their conjugate bases (or anion form3). Carbonic acid (H2CO3) and its conjugate base bicarbonate ion (HCO3-) are the primary buffering system in swimming pool water.
Acids and conjugate bases

When two substances are in equilibrium with each other based on pH, one is the acid, and other is its conjugate base. The only difference between them is a Hydrogen ion. In fact, all acids have a conjugate base.4
The dominant form of alkalinity we have in swimming pools is called carbonate alkalinity. Within the carbonate alkalinity system, carbonic acid is a weak acid, and its conjugate base is the bicarbonate ion. The pH determines the concentration of each.
For any acid and its conjugate base, the pH where they intersect (equal concentration of each) is called the pKa value. This is the pH where their buffering strength is at its maximum. Take a look again at the graph from earlier. Do you see the dashed line at 6.14 pH? This line represents the pKa value between carbonic acid and bicarbonate ion. We'll explain pKa in the next section of this article.
Another example of a buffering system that contributes to total alkalinity is Cyanurate alkalinity.

Cyanuric acid (H3Cy) and its conjugate base (H2Cy-) are components of the cyanurate buffer system. The pKa value for cyanurate alkalinity is specific to this system and can vary depending on the specific buffer components involved. In swimming pools, the pKa for cyanurate alkalinity is approximately 6.88.5
In a non-stabilized pool, chlorine dissolves into its active form (Hypochlorous acid, HOCl) and its weaker form (Hypochlorite ion, OCl-).

The only difference between them is the Hydrogen ion, so HOCl is the acid, and OCl- is its conjugate base. The pKa for HOCl and OCl- is 7.54.
Because of this additional buffering system in our water, increasing CYA impacts the amount of acid needed to reduce pH. You can see this when using the Orenda Calculator™. Cyanurate alkalinity is also subtracted from Total Alkalinity (TA) when calculating the LSI and pH ceiling. All of this math is done automatically for you in the Orenda Calculator™.
A strong acid will have a weak conjugate base, and a weak acid will have a strong conjugate base. There is an inverse relationship between them.
Defining pKa values and their importance
The pKa value represents the pH at which the concentration of an acid and its conjugate base are equal. The pKa value is the pH in which those two compounds have the most buffering capacity. Here's an excerpt from the late renowned chemist John Wojtowicz:
"Maximum buffering occurs at a pH where the molar ratio of acid to anion [conjugate base] is one." 3

On the graphic above, we added a sponge on the right side of the 6.14 pKa value (dashed line) for the carbonic acid and bicarbonate buffering system.
The closer the pH gets to that dashed line, the more resistance it will face. Conversely, if your pool has borates in it, you also have the borate buffering system in your water. See the sponge on the left side of the borate pKa value of 9.2.
As the pH in the pool moves closer to either pKa value, the buffering resistance increases. Conversely, as the pH deviates further from a pKa value, the buffering resistance decreases.
Let's break down pKa.
"p" means negative logarithm, or "-log"
"K" means "constant"
"a" means acid dissociation.pKa = -log(Ka) = negative logarithm of the acid dissociation constant
The Ka value is the degree an acid dissociates into its ions in water. It is a quantifiable measure of the strength of an acid in solution. Thankfully, these values are known for most substances.6
A buffering system's pKa value basically tells us two things:
- pKa tells us the optimal pH for an acid and its conjugate base to buffer against pH change, and
- pKa tells us the strength of an acid. The lower the pKa, the stronger the acid, and vice versa.
pKa versus pKb
Conversely, there are also pKb values. pKb is the same concept as pKa, except it is the base dissociation constant. It tells us the strength of a given base. The lower the pKb, the stronger the base.7
The pKb is the negative logarithm (base 10) of the base dissociation constant (Kb). Kb measures the strength of a base in solution. However, pKb is less commonly used compared to pKa, as acidity is a more prevalent concept in chemistry.
pKb = -log10(Kb) = 10-pKb
The pKa can be used to determine the pKb, because they must add up to 14. This is because the pH scale is from 0-14. If you want to calculate pKb, simply subtract the pKa value from 14.
pKb = 14 - pKa
Equilibrium Between Acid and Conjugate Base
In any buffer system, an acid and its conjugate base are in equilibrium with each other. At their pKa value, they exist in equal concentrations and can readily interconvert ions. When an acid donates a proton (H+), it forms its conjugate base. Conversely, when the conjugate base accepts a proton, it reverts back to the acid form.
The equilibrium between the acid and conjugate base allows the buffer system to effectively regulate the pH by accepting or donating protons as needed.
Conclusion
While pH cannot be controlled, it can be contained using physics. Understanding how buffering systems work helps us manage pH. Too much carbonate alkalinity will have high resistance against acid, but it will also be a source of a higher-rising pH.
We at Orenda advocate for having an appropriate amount of alkalinity based on your primary sanitizer and overall LSI balance needs. Pools primarily using Trichlor tabs need more TA to neutralize the low pH (80-90 ppm TA). Pools primarily using liquid chlorine, cal hypo and salt chlorine generators, by contrast, will be better suited with less TA (60-70 ppm TA) when the water is warmer than 60ºF.
The right alkalinity level allows the pool water to resist drastic pH changes while not being so high that the pH ceiling becomes high enough to cause scale or calcium flakes in saltwater pools.
We hope this lesson helps understand how alkalinity buffers against changes in pH, and how the pKa value determines the strength of the acid involved.
1 Adding acid converts bicarbonate ion into carbonic acid by adding Hydrogen (H+) to it. This is how acids reduce pH in a pool with carbonate alkalinity in it. By converting bicarbonate into carbonic acid, acid re-carbonates the water (think of a flat beer or soda being recarbonated), which increases the amount of dissolved CO2 in the water. The more dissolved CO2, the lower the pH. Learn why here.
2 It should be noted that carbonate alkalinity (the sum of bicarbonate and carbonate ions only) is actually a source of carbonation that causes pH to naturally rise. More carbonate alkalinity means more carbonation and, therefore, more dissolved carbon dioxide to off-gas into the air above the water. That translates into a higher pH ceiling, and therefore a rising pH. Learn more about this in our article about CO2, pH and Henry's Law.
3 Wojtowicz, John. (2001). Swimming Pool Water Buffer Chemistry. Journal of the Swimming Pool and Spa Industry. Vol. 3 (2), pp. 34-41.
4 Bodner Research Group (Retrieved 2024). Acid-Base Pairs, Strength of Acids and Bases, and pH. Purdue University Chemistry Department.
5 National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 7956, Cyanuric acid.
6 pKa values for any acid and conjugate base pair can easily be found online, just like the thermodynamic constants and molar weights of the known elements and chemical compounds worldwide. We can thank scientists who have paved the way for us to understand these things over hundreds of years.
7 Lower, Stephen. (Retrieved 2024). Relationship between Ka, Kb, pKa, and pKb. Chemistry LibreTexts. Ch. 7.12.
