What is the ideal pH for swimming pools?

Traditional pool industry teachings tell us that maintaining the pH within the ideal range of 7.4 to 7.6 is crucial for swimming pools. But why? And is there actually an ideal pH in the first place? Let's find out.
Covered in this article:
- What is the ideal swimming pool pH?
- Water and air physics in harmony
- Water equilibrium (LSI)
- Air equilibrium (Henry's Law)
- pH cannot be controlled, but it can be contained
- Why does pH matter in pools?
- pH and chlorine efficacy
- pH and corrosion/scaling
- pH and swimmer comfort
- My opinion, as a swimmer
- Conclusion
What is the ideal swimming pool pH?

pH impacts just about everything in water chemistry. Indeed, pH is based on water itself (H2O). The pH of water determines many things:
- Carbonate alkalinity types present in water,
- Chlorine efficacy (speed, strength, %HOCl) in non-stabilized pools,
- Water balance (Langelier Saturation Index, (LSI)).
The ideal pH of a swimming pool depends on what you're trying to accomplish in both water quality and water balance. Water quality is disinfection, cleanliness, and clarity. Water balance is a function of physics, which we measure using the LSI. Furthermore, pH cannot be controlled, so picking a specific target (like 7.4) is temporary, at best. It can be a helpful practice, however, as long as you understand that the pH will naturally rise from that point up to its pH ceiling. More on that in a moment.
No matter where your swimming pool is located, the ideal pH range for your pool should optimize both water quality and water balance. And based on our research and field experience, the most practical way to accomplish both is by embracing physics instead of fighting them.
Water and air physics in harmony
Everything in nature seeks equilibrium. Our ecosystem has its own natural checks and balances, and that includes water and air. Left alone, a swimming pool will revert to its natural state. Think about it...what would your pool look like if you left it alone for months? Probably something like this...

As you can see, a neglected swimming pool looks a lot like a pond. If you were to test the water balance of a natural body of water, like a pond, you would find the water is LSI-balanced. Balance is water's natural state.
But from a water quality standpoint, this pond is clearly unsafe to swim in. We need water to be safe, clean and clear, which means disinfected, filtered and circulating. Disinfection is not natural for water.
We as pool owners and operators must walk a narrow path of both giving water the LSI balance it craves, and keeping the pool sanitary and clean 24/7. These two goals do not need to be mutually exclusive! We can do both––and should do both––when we understand the water and air physics involved.
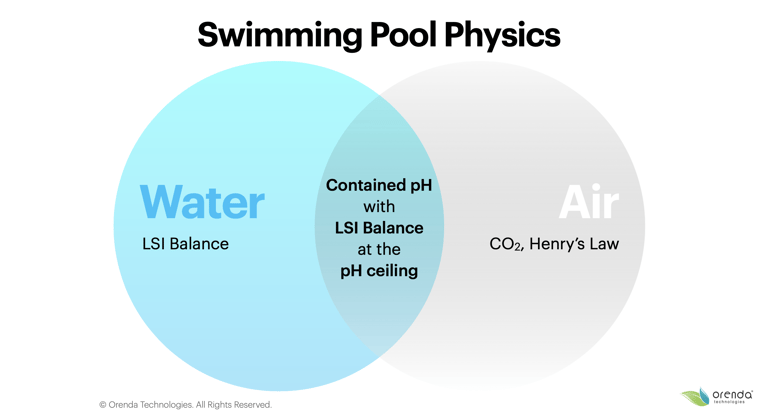
So, what are these physics, exactly?
We can distill them down into two concepts. The first is about water: water seeks its own equilibrium of calcium carbonate saturation. This is measured using the LSI. The second concept is about air: air seeks its own equilibrium with gases dissolved in water.
Water equilibrium (LSI)
We have written extensively about the Langelier Saturation Index (LSI) all over our site. Just search it on our site to read more. In short, water craves a certain saturation of calcium carbonate (CaCO3).
Like anything else dissolved in water, if there is too much, water becomes oversaturated and cannot hold more (think of too much sugar in a drink, sinking to the bottom). If there is too little calcium carbonate saturation, water will be aggressive and hungry for it. Low-LSI water will dissolve calcium anywhere it can find it (plaster etching), and if calcium is unavailable, it can attack non-calcium surfaces like vinyl liners and fiberglass pool surfaces too.
Either we give water the LSI balance it craves, or it will have to balance itself. While we have countless options to balance water, water itself has only two: eat or scale.1
Air equilibrium (Henry's Law)
Open a soda, beer, or other carbonated drink, and you will hear pressurized CO2 escaping when you crack open the lid. Before opening the lid, the sealed drink had no visible bubbles in it. But the moment the lid opens, bubbles appear and start floating to the top. This is an example of Henry's Law of the Solubility of Gases.
Henry's Law basically states that any gas dissolved in a liquid must be directly proportional to the partial pressure of that gas above the liquid.2 In other words, any gas dissolved in a liquid must equalize with that same gas above the liquid. We have another article that focuses on why Henry's Law causes the pH to rise in swimming pools.
The point is this: because we have carbonate alkalinity in our water, our water is more carbonated than the air above it. Therefore, our water must lose CO2 until the CO2 is in equilibrium with the air above the pool. It's the same physics that makes a beer or soda go flat over time. Pools are trying to go flat too. And when they do, the pH cannot rise any higher. Call this threshold the pH ceiling.
pH cannot be controlled, but it can be contained
Thanks to Henry's Law, our pH rises with the natural loss of CO2. So instead of trying to control pH, let's instead focus on containing pH.
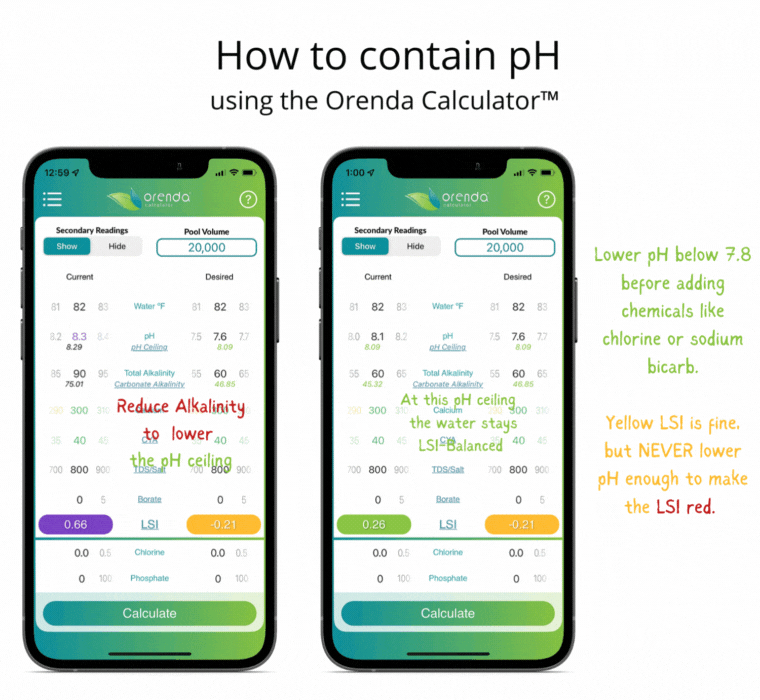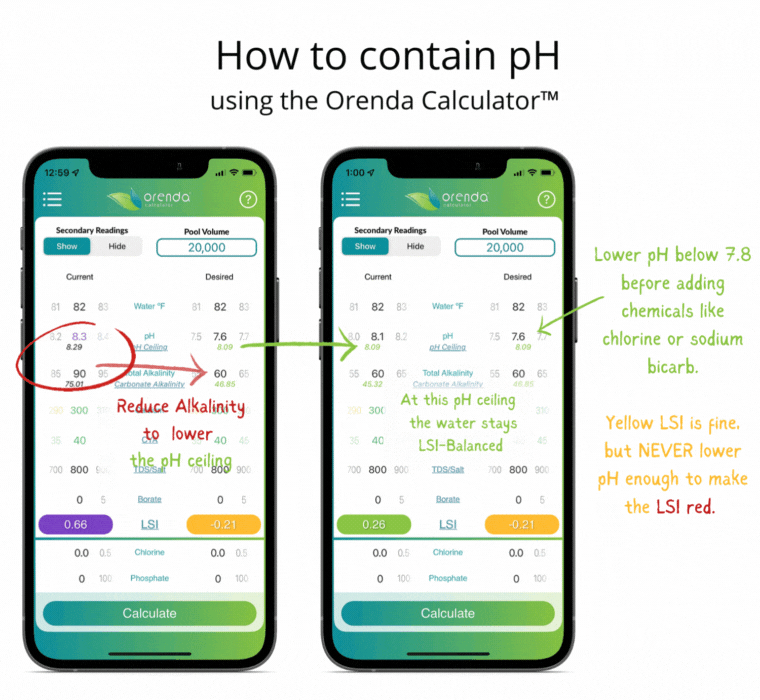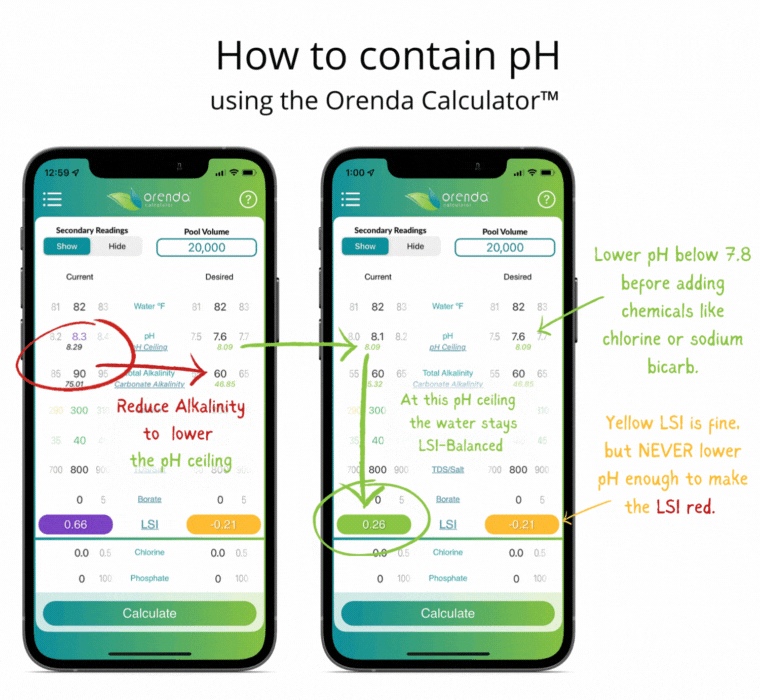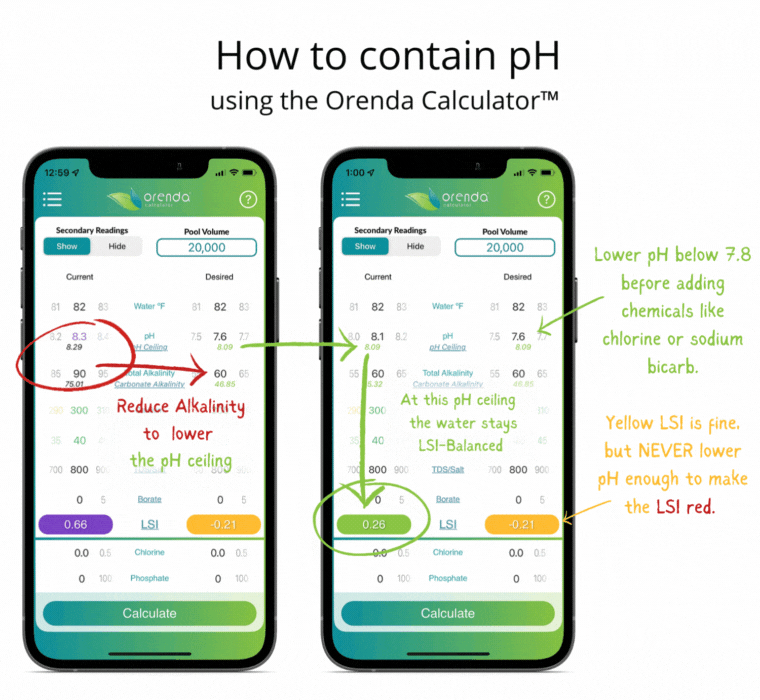
We can select how carbonated our water is by determining the carbonate alkalinity. This is done by selecting a total alkalinity level and CYA level that give us a favorable pH ceiling.3 This is why we recommend containing pH instead of trying to control it.
Why does pH matter in pools?
If we cannot control pH, and we know it's going to rise naturally up to its ceiling, why does it matter so much? Why are we told to maintain 7.2 to 7.8 pH, and ideally 7.4 to 7.6?
Valid question.
The answers are somewhat complex, because there are many factors at play. There are the desired results for water quality and balance, of course, and there are also the competing interests at play during the process of creating the standards themselves. So let's examine each of the three reasons our industry is told we need to keep water within 7.2 to 7.8 pH.
- pH and chlorine efficacy
- pH and corrosion/scaling
- pH and bather comfort
pH and chlorine efficacy
There is no doubt that pH has a significant impact on the strength, speed and efficacy of chlorine. Look at the chart below. The red line shows that lower pH means a higher percentage of Hypochlorous Acid (%HOCl), which is the active killing form of chlorine. The smaller that percentage, the higher the percentage of the much weaker form of chlorine, Hypochlorite ion (OCl-). Therefore, the higher the pH, the weaker the chlorine.
The graph on the left is the standard equilbrium of free chlorine relative to pH. The red line is HOCl, and the green line is OCl-.
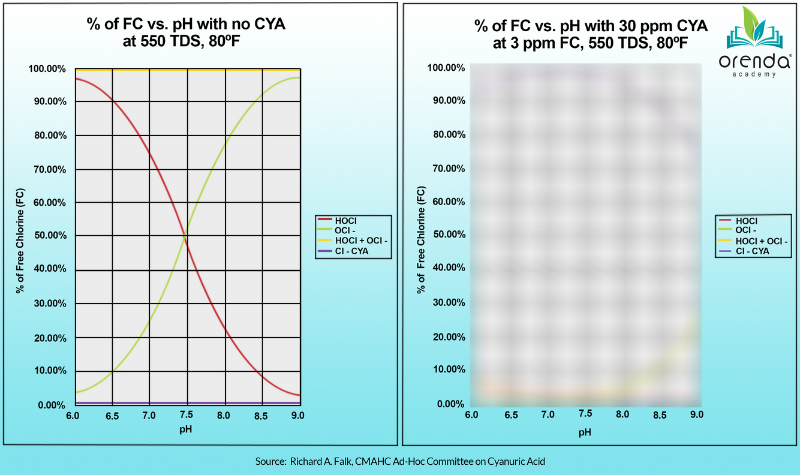
The chart on the left is true, and we are not disputing it. But here's the catch: this chart only applies to water without cyanuric acid (CYA) in it. When CYA is introduced, the relationship between pH and chlorine fundamentally changes. Look at the chart on the right, below. Where's the red line (%HOCl) now?
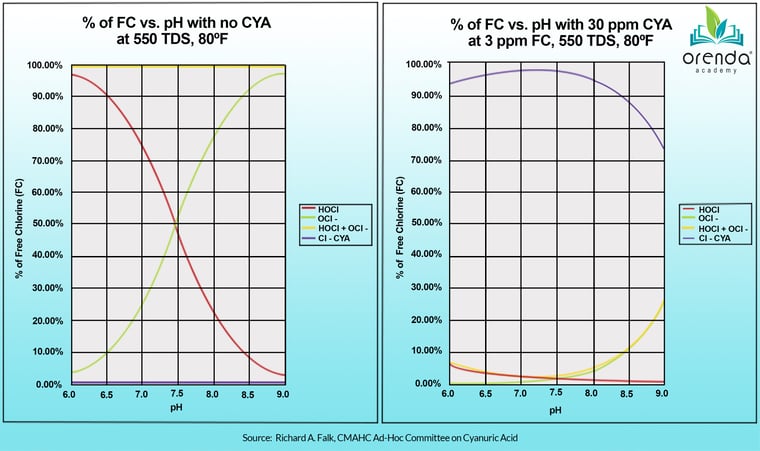
As you can see, there is not much difference in %HOCl as the pH moves. Here's another chart showing the same thing:
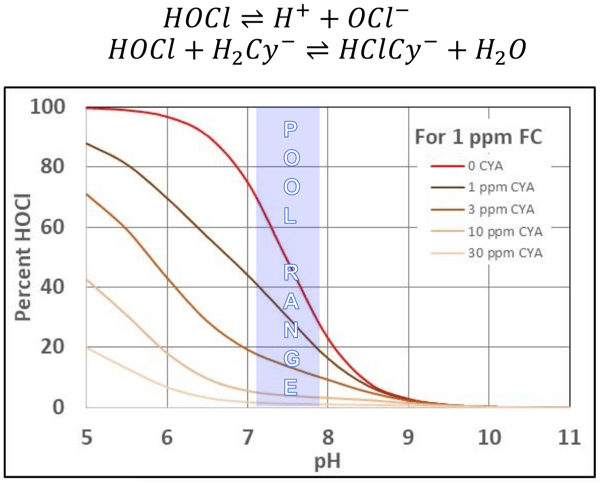 Source: Cyanuric Acid - CMAHC Ad Hoc Committee Report, Slide 6. - Richard A. Falk
Source: Cyanuric Acid - CMAHC Ad Hoc Committee Report, Slide 6. - Richard A. Falk
This point has also been studied and published by Dr. Stanley Pickens and several others. So for indoor pools without CYA, pH makes an enormous impact on chlorine efficacy––like indoor pools. On stabilized pools, however, pH makes a much smaller impact on chlorine strength and speed. What does matter, however, is how chlorine (OCl-) will separate from CYA at higher pH levels. You can see this in the bottom right-hand corner of the right chart earlier. We elaborate more on this concept of chlorine loss here.
So if it's not pH that drives chlorine speed and efficacy in stabilized pools, what does?
The amount of cyanuric acid does. Specifically, the ratio between free chlorine and cyanuric acid (FC:CYA). Learn more here.
Water safety is still the top priority
We understand and respect why health departments, standards and codes enforce having a pH below 7.8 on public/commercial swimming pools. Their intentions are pure: we DO need effective chlorine to keep water safe, clean, and clear. Nobody wants people getting sick from a Recreational Water Illness (RWI), especially not us.
We totally agree with the goal of having safe, disinfected water. And for nonstabilized pools, the standards are fine. The issue we bring up is that the pH does not impact the chlorine in the same way when CYA is in the water––and CYA is in the vast majority of outdoor pools in the world. The chart the standards are based on simply do not apply to stabilized pools, and the science seems clear about that.
In our opinion, what we should really be focused on in stabilized pools is the ratio of CYA to free chlorine, because that is what determines the contact time (CT) for given pathogens. The US Centers for Disease Control (CDC) acknowledges the need for minimal CYA because they, like us, want optimal disinfection in water.5 To reiterate, we care deeply about the health and safety of swimmers, and we want chlorine to be effective in water.
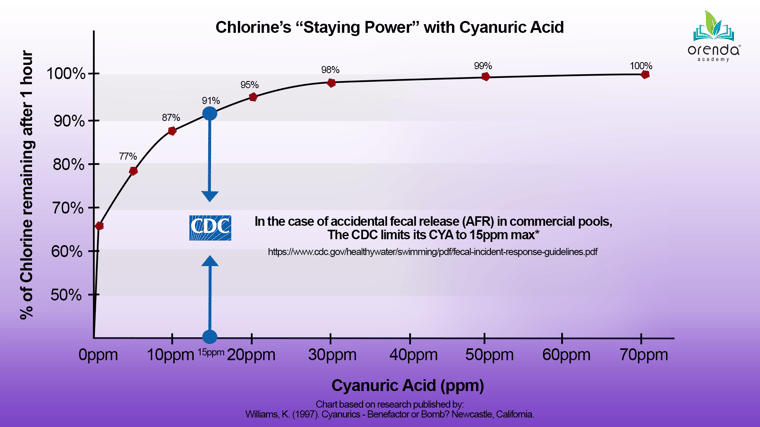
Chlorine strength is probably the most common argument we hear when it comes to choosing an ideal pH, but as mentioned earlier, it is not the only one. The next one is...
pH and corrosion/scale
pH directly impacts corrosion and scale formation. There are several other factors at play for corrosion, including TDS (conductivity), electrochemical reactions, oxidation and chemistries like sulfates and chlorides. Metal corrosion is highly pH dependent, as it has to do with the concentration of carbon dioxide and oxygen in the water.6
When we talk about corrosion in the swimming pool industry, however, it is a generic term that also includes other damage, like etching. Etching is basically the corrosion of cement. Aggressive water dissolves the calcium out of it, causing a deleterious reaction where material is lost. The pH matters for this, but etching is primarily a function of a low LSI, where pH is just one of six factors.
So while some metal corrosion may not be determined by the LSI, cement etching certainly is. And if the LSI is low (below -0.30), the probability of any type of corrosion increases.
Calcium carbonate scale formation, however, is entirely driven by LSI balance. Yes, pH matters, but it matters within the context of the LSI. In other words, pH is not the only thing that drives scale formation. A high LSI (above +0.30) does.
The third justification for maintaining 7.2 to 7.8 pH is...
pH and swimmer comfort

Photo licensed from Shutterstock.com
Myth: The pH of the water should be 7.4-7.6 because human tears (and eyes) have a pH of 7.4.
Truth: Human tears can range from 6.5 - 7.6 pH.7
There's actually quite a bit of research on the pH of our eyes and tears. According to the studies we have read, people's eyes tend to get more alkaline through the day, and the pH of tears also depends on how hydrated someone is.8 The point is, eyes do not always have a pH of 7.4. And is pH really the driving factor behind bather comfort and discomfort?
In my experience, no.
Bottled drinking water brands vary in pH from below 5.5 to over 9.5. Keep in mind, pH is logarithmic. 5.5 water is 10,000x more acidic than 9.5 pH water. And yet we can drink them both and hardly taste the difference.
Hopefully nobody is deliberately maintaining pools below 5.5 or above 9.5 pH.9 We're not talking about those extremes in the first place. We're talking about pH naturally rising up to its pH ceiling, which is usually between 8.0 and 8.2 in normal conditions.
My opinion, as a competitive swimmer
Will an 8.2 pH irritate us? No, I don't think so. The bather comfort argument is just one of many misunderstandings about pH. I personally do not think pH is the leading cause of eye and skin irritation in swimming pools. That title belongs to chlorine byproducts like chloramines. The same can be said for bromine pools (bromamines). Chloramines and other DBPs burn our eyes, throats and lungs; they irritate our skin and cause discomfort.
Conclusion
Is there even such a thing as an "ideal pH"? If so, it would take both chlorine efficacy and LSI balance into consideration. And since pH moves regularly, naturally rising up to its pH ceiling, is it even practical to pick one number and call it "ideal"?
This is why we recommend a containment strategy in private residential swimming pools, not a strict pH range or target. We have color-coded desirable pH ceiling values as green on the Orenda Calculator.10 On public/commercial pools, the pH maximum of 7.8 must be observed until health departments change the standards. Remember that disinfection is the top priority (as it should be). Standards like these do not change overnight, and the first step is education.
1 The LSI formula requires six factors to calculate (and seven if borates are in the pool). To find the number of possible combinations of six numbers, we must factorial 6 (6! on a calculator). That's 6x5x4x3x2x1 = 720 possible combinations. If you use borate as a 7th factor, that jumps up to 5,040 combinations. Multiply that by the number of options on each dial on the Orenda Calculator™, and we have millions of ways to balance the water ourselves. And yet, water can only balance itself one of two ways: eat or scale. If the LSI is below -0.30, water will dissolve calcium (eat) until it is back within LSI balance. If there is an oversaturation of calcium carbonate, the LSI will be over +0.30, and water will have to get rid of some calcium, which precipitates out of solution as scale. LSI is so important that it is our first pillar of proactive pool care.
2 Sander, R. (2023). Compilation of Henry's law constants for water as solvent. Atmospheric Chemistry Division, Max Planck Institute for Chemistry. 23, 10901-12440, doi:10.5194/acp-23-10901-2023
3 "pH ceiling" is a term we have coined. It is an easier name for pH(eq). It is the pH of water when the CO2 in the water has equalized with the air above the water, and therefore no more CO2 can be lost through natural aeration. If no more CO2 can be lost, the pH cannot naturally rise higher...though it can be forced if mistakenly do things like overdose acid (which etches cement and pulls out a high-pH calcium hydroxide) or add soda ash (which changes the carbonate alkalinity and rapidly raises pH).
4 Bishop, M. (2013). pH and Equilibrium. An introduction to chemistry by Mark Bishop. Chiral Publishing Company.
5 US Centers for Disease Control. (2018). Fecal incident response recommendations for aquatic staff. Healthy Swimming fact sheet.
6 Water Quality Association. Corrosion.
7 Abelson, M. B., Udell, I. J., & Weston, J. H. (1981). Normal human tear pH by direct measurement. Archives of ophthalmology (Chicago, Ill. : 1960), 99(2), 301.
8 Coles, W., Jaros, P. (1984). Dynamics of Ocular Surface pH. British Journal of Opthalmology. 68, pp. 549-552.
9 Sure, extreme circumstances can take pool water outside those bounds. Think hot starts that force pH below 4.3 to zero out alkalinity, and spiking pH during startups can get over 9.5, as can algae that consume CO2.
10 To see the pH ceiling in the Orenda Calculator™, tap "show" secondary readings on the toggle at the top left. For more info, read our help center.
