What is Carbonate Alkalinity?

The LSI calls for the carbonate alkalinity of pool water...but this is a confusing term. Pool chemistry test kits only measure Total Alkalinity (TA). So what is Carbonate Alkalinity? And how is it different from TA?
Covered in this article:
- What is carbonate alkalinity?
- Carbonate ions (CO32-)
- Corrected alkalinity
- How to calculate corrected and carbonate alkalinity
- Corrected alkalinity equation
- Alkalinity buffering system
- Bicarbonate and Carbonate contain CO2
- Conclusion
What is carbonate alkalinity?
We will start by venting some frustration. Two different things are both called "carbonate alkalinity" in water chemistry:
- Carbonate ions (CO32-), and
- both Bicarbonate (HCO3-)and Carbonate ions together (aka "corrected alkalinity").
Carbonate ions
Once the pH reaches 8.3, bicarbonate ions (HCO3-) drop their Hydrogen and become carbonate ions (CO3--). This is shown on the alkalinity equilibrium chart here:
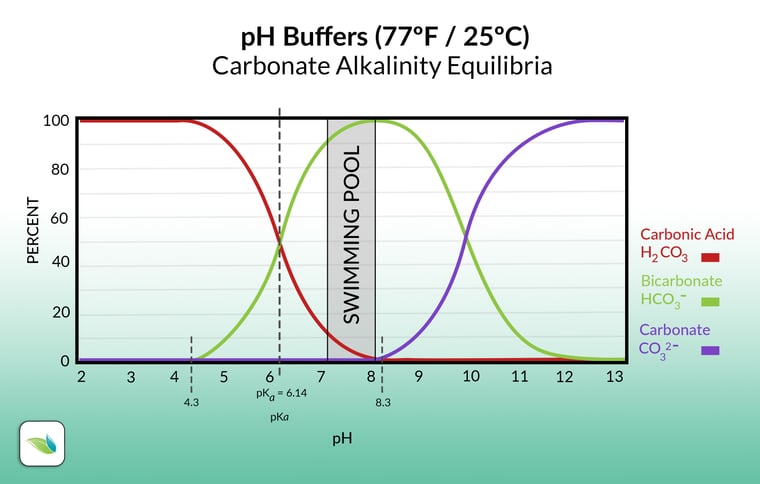
The purple line shows carbonate ions, which begin to appear at 8.3 pH, when bicarbonate ions start converting.
As you can see from the chart, in the pool chemistry pH range, the vast majority of alkalinity is bicarbonate. Carbonate ions begin to form only if your pH exceeds 8.3. This explains why it's so much more difficult to keep calcium in solution over 8.3 pH: carbonate ions are very attracted to calcium, so they create calcium carbonate and precipitate out of solution:
Ca++ + CO3-- ⇌ CaCO3
Calcium + Carbonate ion ⇌ Calcium Carbonate
Think about plaster dust, carbonate scale in salt cells, or soda ash clouding up the pool. These conditions occur because of localized LSI violations. Plaster dust has 12.6 pH calcium hydroxide forcing carbonate ions to form; salt cells produce a byproduct of 13.5 pH sodium hydroxide, which forces carbonate ions to form; and soda ash itself is sodium carbonate with a pH of 11.6. All three precipitate calcium carbonate thanks to the reaction above.
Related: LSI Balance and Calcium Management (Pillar 1)
Corrected alkalinity
Corrected alkalinity refers to both bicarbonate and carbonate alkalinity, but nothing else. This "correction" means subtracting cyanurate alkalinity (and borate, if you use them in your water).
Our free Orenda App automatically does all the math for you. Simply input your water's pH, total alkalinity and stabilizer (CYA) in the calculator, and the LSI value will adjust accordingly. But for the sake of education, we cover how to calculate corrected alkalinity later in this article.
It is corrected alkalinity that most swimming pool people think of as "carbonate alkalinity", thanks to the LSI formula. But technically speaking, carbonate ions are just one subset of that group, and they are technically called carbonate alkalinity. The semantics are so annoying it's obnoxious.
Related: What is Alkalinity?
How to calculate carbonate alkalinity
Corrected alkalinity equation
To calculate corrected alkalinity, simply subtract cyanurate alkalinity (and borate, if applicable) from total alkalinity1. The cyanurate alkalinity factor is roughly one-third of your CYA ppm, which then must be subtracted from TA. If you want to be more precise, the exact correction factor depends on pH. The lower your pH, the smaller the correction factor. See the LSI chart below:
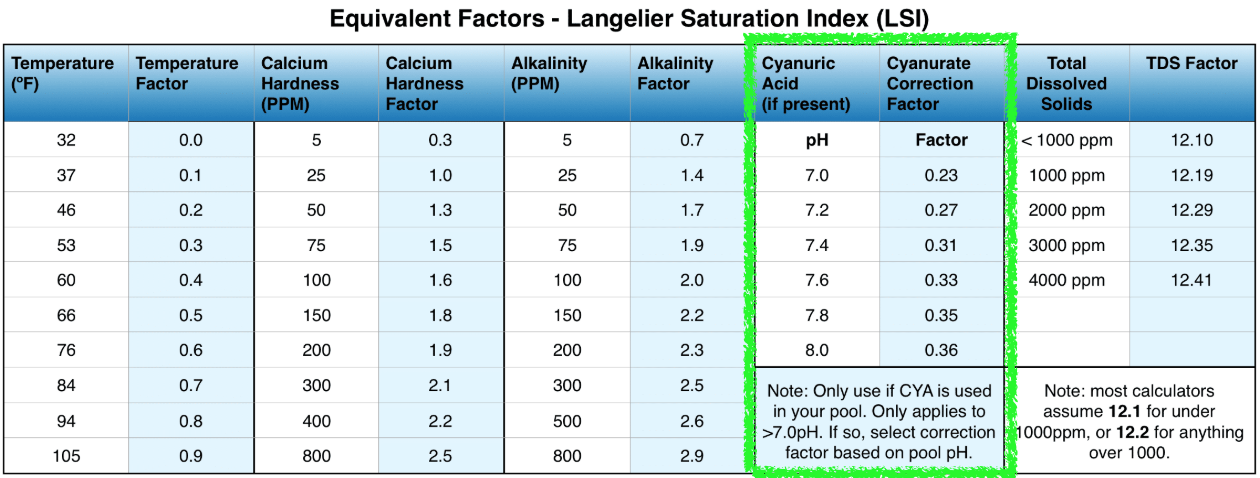
Let's do an example. Say you have 7.8 pH, 100 ppm total alkalinity, and 60 ppm CYA:
Corrected Alkalinity (CA) = Total Alkalinity (TA) - (CYA x [correction factor @ pH])
CA = 100 - (60 x [correction factor @ 7.8 pH])
CA = 100 - (60 x [0.35])
CA = 100 - (21)
CA = 79 ppm
As you can tell, raising CYA increases the correction,2 which means it lowers the corrected alkalinity. That means higher CYA means lower LSI. This is one of the reasons why minimal CYA is our fourth pillar of proactive pool care. Of course, if your pool does not use any CYA, total alkalinity should be all carbonate (with very few exceptions). So in a non-stabilized pool, there is no need for corrected alkalinity. Just use your TA.
And again, the Orenda Calculator™ does all of these corrections for you.
Alkalinity buffering system
One of the most common questions we get is the difference between pH and total alkalinity, because both need to be tested on a weekly basis. They are related in that alkalinity buffers against changes in pH; specifically against decreasing too rapidly. This essentially means that all types of alkalinity can either receive or give a Hydrogen ion (dissociation in both directions). Here are the equilibria of the carbonate buffering system with dissociations:
CO2 + H2O ⇌ H2CO3 ⇌ HCO3- + H+ ⇌ CO3-- + 2H+
Carbon Dioxide + Water ⇌ Carbonic Acid ⇌ Bicarbonate Alkalinity + Hydrogen ⇌ Carbonate Alkalinity + 2 Hydrogen
Bicarbonate and Carbonate contain CO2
 Because alkalinity can both give and receive Hydrogen, it slows the change in pH. We know the amount of CO2 in your water determines the pH, thanks to physics. The more CO2 in solution, the lower the pH, and vice versa.
Because alkalinity can both give and receive Hydrogen, it slows the change in pH. We know the amount of CO2 in your water determines the pH, thanks to physics. The more CO2 in solution, the lower the pH, and vice versa.
So you might be wondering why muriatic acid (HCl) lowers pH and alkalinity since there is no CO2 in muriatic acid. The answer is simple: acid adds hydrogen, which reverses the dissociations shown in the equilibria above. Bicarbonate and carbonate ions contain CO2, and acid converts it into carbonic acid, which lowers the pH. This is why alkalinity is also reduced with acid: it was sacrificed. Take a look at the graph above and you can see what happens when you move left along the X-axis.
If you want to circumvent the reduction in alkalinity, many pools lower pH by injecting carbon dioxide into their water.
Cyanurate alkalinity does not contain carbon dioxide. Neither does borate3. Therefore they need to be removed (corrected) from total alkalinity so the LSI knows how much carbonate/bicarbonate alkalinity is actually in the water. But again, if you don't have any CYA or borate in your pool, you have no need for alkalinity correction. The LSI formula would use your total alkalinity reading.
Conclusion
We know this was a dense topic, and we hope we were able to reduce confusion. But let's be honest here...it's complicated. The reason it's important to know the corrected alkalinity is because other contributors to total alkalinity (like cyanurate alkalinity and borate) do not contain carbon dioxide. And because they have no carbonate in them, they do not apply to the LSI.
So when it comes to the saturation of calcium carbonate, which is what the LSI measures, we need to account for those other types of alkalinities and remove them from the equation. If you want to learn more, we encourage you to do more research on your own. You can start here, here and here. If you have questions for us, please ask us in the comments below.
1 Borate is another pH buffer that must be subtracted from total alkalinity if used. That being said, borate's LSI impact is minimal compared to CYA's impact.
2 This white paper from John Wojtowicz is among the best resources on this topic in existence. If you are looking for cyanuric acid information and how it buffers pH, this article has it all. It was our primary source for this article.
3 Wojtowicz, John A. 1993. The Effect of Cyanuric Acid and Other Interferences on Carbonate Alkalinity Measurement. Page 60.
