How to Open a Pool with Crystals in it

What are you going to do if you remove a winter pool cover and find calcium crystals in the pool? If you have encountered winter crystals in the past--and you may have thought it was 'scale'--what did you do in the past? Did it work? This article will outline four things Orenda recommends if you are trying to open a pool with crystals in it.
Covered in this article:
- Calcium crystals are NOT scale
- Crystals can be prevented by proper winterization
- How to open a pool with crystals in it
- Conclusion
Calcium crystals are NOT scale
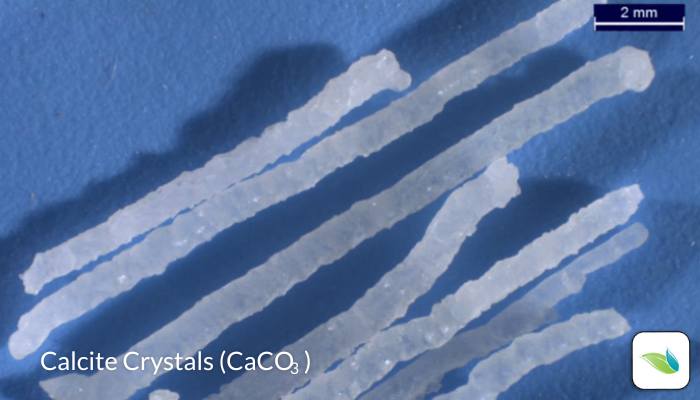
From our lab studies of several samples of swimming pool crystals, we have established they are made of "skeletal calcite", which is a crystalline form of calcium carbonate (CaCO3). But just because they are calcium carbonate does not mean they are a type of scale. Calcite crystals are actually the opposite of scale.
The problem here is more than just semantics. If we as pool people look at any form of calcification and just reflexively call it "scale", we run into issues. And that's what has happened for years with this crystals phenomenon. Everyone has thought these crystals are scale, and therefore treat them accordingly. We have seen too many pool companies and homeowners deploy scale-fighting habits against this issue. Habits like keeping calcium levels low and forcing pH to stay down all year long.
The real problem that causes these crystals is a low LSI during the winter; usually driven by low calcium hardness and cold water temperatures.
Indeed, thanks to lab testing and abundant empirical evidence from the field, we know that calcite crystals grow out of cement. Calcium carbonate scale, on the other hand, originates from an oversaturation of CaCO3 in the water, which precipitates out of solution, and lands on surfaces. If these crystals were scale, they would be on top of non-cement surfaces like plastic fittings, handrails, and tile. But they are not.

.jpg)
.jpg)



.jpg)
.jpg)



Swimming pool crystals can be prevented by proper winterization
If you winterized your pool properly, with the LSI in mind, you should never have calcite crystals in your pool in the first place. This is as simple as using the Orenda Calculator™ and looking at the coldest possible temperature your water might encounter during the winter. When your pH is at its ceiling, is your LSI balanced at that coldest temperature? If not, make it so.
Related Procedure: How to Winterize a Swimming Pool the Orenda way
If you fail to prepare the water for cold temperatures, some form of damage is bound to occur when the LSI drops. If you have a vinyl liner, expect some fading. Fiberglass pools may degrade, and eventually turn white when chlorine oxidizes the gel coat (we call this "chalking"). Plaster and other cementitious finishes either get crystals or some other form of calcium issue, like winter dust or what we call "ghosting". Sometimes it's a combination of several things.
So prepare your water for winter ahead of time, because we hope you will never need to read this article again. 👍🏼
How to open a swimming pool with crystals in it
So what do you do when you open a pool that already has crystals in it? Follow these four steps.
4. Arrive at the pool prepared for crystals
Obviously, we hope the pool doesn't have sharp calcite crystals in it, but hope is not a strategy. Instead, assume the worst and have the tools and chemicals to handle crystals with you on the truck. These items include:
- test kit
- clean white bucket
- two measuring cups (for liquids and dry chemicals)
- thermometer
- calcium chloride
- sodium bicarbonate
- muriatic acid
- SC-1000 Scale & Metal Control
- wire brush
- diamond pad (sanding pad)
- smartphone with the Orenda App and a camera
A pool professional should have all of these in your service vehicle regardless of the time of year, but it's especially important during spring openings.
3. Collect a water sample, warm it, and test the water chemistry
It should go without saying that you need to know the water chemistry when you arrive. But simply testing cold water will not yield accurate results. So collect a water sample in a clean bottle, then warm it up. One way to warm up the sample is to put it in your jacket or pocket, next to your body. You can also put it in your truck and let it warm up in the sun. As long as the bottle is closed and you are not contaminating the sample, you can be creative here, just warm up the sample above 60ºF. We say 65ºF to be safe. Most test kits are not accurate with water below 60ºF.
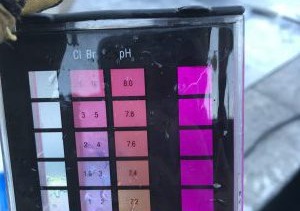
While you are warming the sample, you can use your thermometer to test the actual water temperature in the pool. Input the temperature into the Orenda Calculator™. You will need it for the LSI.
Once your sample is warmed, test for every LSI factor: pH, alkalinity, calcium hardness, CYA and TDS. You already know the water temperature from the thermometer. Plug all these values into the Orenda Calculator™ and you will have the LSI of the water. Chances are it will be a purple number (high LSI) due to a sky-high pH.
Why the LSI might be high when you test it
Do not be fooled by the LSI value you are looking at. You are measuring the LSI today, which is not the LSI you had during the winter when the crystals formed. The crystals formed due to a red (low LSI) condition. But the water had to bring itself back to equilibrium, so it pulled calcium out of the cement, which spiked the pH and increased the calcium hardness level slightly.
We would love to run tests with real-time pH probes during the winter on a pool to see when (and how fast) this occurs.1 We suspect the water begins to eat the moment the LSI dips below -0.30, and the pH continues to rise as it eats. But at some point, we know the LSI stabilized itself in the coldest temperature. By the time you get to the pool, the water is likely 20 or more degrees warmer than it was in the dead of winter. That rise in temperature raises the LSI from where it was.
Related article: Cold water and the LSI
2. Collect samples of the crystals
While we have already done lab tests and have a pretty good idea of what these crystals are, our industry can benefit from more data. Some of the industry associations have teamed up to fund lab testing of these crystals. So if you have crystals, please collect them in a plastic bag and drain the water out of the bag. Mail them to NESPA following these instructions.
Samples can be sent to the lab for analysis. And who knows? We may find a fifth type of crystal that we have not yet encountered.2 So please send in the crystals, and let them know you heard about it from us. That way we can follow up on them and learn from the crystals too.
1. Balance the LSI and purge with SC-1000
Finally, once you arrived prepared, tested the warmed sample of water, and collected samples of the crystals, it's time to correct the water. This is done in two easy steps, then brush the entire pool with a wire brush.
The priority is to balance the LSI. We know that the crystals were caused by a low LSI, so it's time to balance the LSI and get enough calcium into the water. It sounds counterintuitive, but adding calcium is a necessary part of this process. If you think about it, it makes sense. The water has been starving for it.
How to balance the LSI when there are crystals in the pool
You presumably will be looking at a very high pH and moderate or low calcium hardness. Total alkalinity can vary, and so can your other factors. So let's focus on pH and calcium hardness for a moment. You probably need to increase calcium and lower pH simultaneously. Use the Orenda Calculator™ to find out exactly how much calcium chloride and acid you will need. Mix the calcium and acid in buckets of water together, and add some SC-1000 to chelate the calcium you are adding. Follow this procedure. Do not add the buckets to the pool until the liquid inside is completely clear.
The alkalinity you have will determine how rapidly you do this. If your alkalinity level is over 100 ppm, you may be able to add acid more rapidly. If you have low alkalinity, dilute the acid and calcium more and spread it around the pool making sure it stays in solution.
The important thing is to build a foundation for LSI balance for the rest of the season. You will likely need to increase calcium again in the fall before winterizing again.
Purge with SC-1000
In fairness, purging with SC-1000 in the spring is not unique to pools with crystals; we recommend purging every pool in the spring with SC-1000, CV-600 or CV-700 enzymes, and PR-10,000 phosphate remover.
Of the four distinct types of calcite crystals we know of, SC-1000 alone seems to only break down two of them. But together with balancing the LSI (as we must mentioned earlier), calcite crystals tend to soften and go away. At the very least, they should be easier to remove with a wire brush over the course of two or three weeks. The warmer the water, the faster this process works, because SC-1000 activates better in warmer water.
If your crystals are not softening or going away after a week of use, it may be a type of crystal that is more stubborn. In that case, unfortunately, you may need to drain and manually remove them. We have seen plenty of pools with crystals that did nothing when directly acid washed. It is a strange (and vicious) phenomenon.
Conclusion
The best defense against crystals is a good offense. Winterize your pools proactively in the fall, with the LSI in mind. Such a strategy involves preparing for the winter months by adjusting your calcium, alkalinity, and pH in preparation for cold water. We also suggest periodic winter visits to continue to adjust LSI.
The type of pool safety cover you use also matters. Mesh covers allow for CO2 to escape, so your pH will reach its pH ceiling. But mesh covers also let rain and snow dilution in, which lower calcium hardness levels. So be prepared for that by loading up the pool with extra calcium hardness going into the winter.
If you DO have a pool with crystals, following the four steps outlined above should help you rectify the problem as well as you can. Unfortunately, the damage is done, but this four-step procedure should help you mitigate it further. Let us know if you need help in the field.
1 Such an experiment would be great, but it takes bandwidth and a time commitment that we alone do not have these days. It's just another 'wish list' item, along with tracking real-time pH during our startup compared to other startups. Eventually, we hope to get to this. We think the data would be compelling. It's not urgent, however, because we know enough to prevent this problem, which is what really matters.
2 We currently know of four types of calcite crystals. We believe there are more. And these four do not include calcium sulfate scale, which forms in sharp crystals.

.png?width=760&height=428&name=SC-1000%20(1).png)