Calcium Crystals vs. Scale

If you have ever opened a winterized swimming pool in the spring and found calcification on the walls and floor, it is most likely not scale. The odds are it's actually calcite crystals in your pool. This article will explain the differences between scale and crystals, as well as how to treat and prevent the crystals from coming back.
NOTE: This article has been revised from its original version due to learning more from laboratory results. We still have more unanswered questions about crystals than we have answers. Why do they harden? How many forms are there (we know of at least 3)? Why are some crystals easy to clean up, and others stubborn? What common denominators are involved, beyond cold water and lack of calcium hardness?
Covered in this article:
- How we first learned about calcite crystals
- Understanding calcium carbonate
- Calcium carbonate saturation in water
- Calcite crystals vs. calcium carbonate scale
- What are calcite crystals?
- What is calcium carbonate scale?
- How to distinguish between scale and calcite crystals
- Four types of calcite crystals in swimming pools
- Treatment: do's and don'ts
- Conclusion
.jpg)



.jpg)

.jpg)




.jpg)

How we first learned about calcite crystals
Let's start with some history. In 2017, we were in the Northeastern United States making sales calls and visiting customers. We kept hearing of these horror stories about "scale" when they would open their pools in the spring. It seemed like everyone in the pool business had a story to tell about it.
At the time, we had recently released the Orenda Calculator™, and we were learning about the LSI ourselves. Something didn't make sense. Low temperatures and low calcium hardness lower the LSI...but calcium carbonate scale is a high-LSI violation. We soon learned that semantics matter, because calling this stuff "scale" was leading to horrible winterization behavior. Because people assumed the calcium they were seeing in the spring was scale, they had developed habits of reducing calcium via dilution in the fall. The thought was less calcium must lead to less risk of "scale" in the spring.
As it turns out, the "scale" was not scale at all. It was the opposite problem. Every single case was calcium crystals. Here are some common factors in all of the cases we encountered:
- In each of the pools' water chemistry during the winter, the LSI was very low. Like -1.20 and below. It was severe.
- Each customer was using low pH (acidic) products to combat the problem.
- The water was cold. These crystals were formed during the winter and discovered during spring openings.
- None of the pools were circulating during the winter. The water was still and quiet for months.
- These deposits were forming underwater, below the winterization waterline. None of this was present above the waterline, on tiles, lights, plastic fittings, or even pebbles in the surface. The calcium crystals were only forming on the cement itself. Since scale falls out of solution and lands on any surface, this proved fact what we were looking at could not be scale.
All these observations told us that this calcium had not come out of solution (scale). It had grown out from the plaster. This led us to start doing research. At first, we thought it was calcium hydroxide because we know that calcium hydroxide bleeds out of plaster when it's curing. As it turns out, we were half right about that. The calcium hydroxide in the cement is absolutely involved, but as it is drawn out from within the cement, it is carbonated into skeletal calcite, which forms the crystals.
Understanding calcium carbonate
The first step in understanding the differences between scale and crystals is to understand calcium carbonate itself. Calcium carbonate (CaCO3) is the most abundant form of calcium on earth. It is the building block of limestone, and it is also called calcite.
On the periodic table of elements, the 20th element is calcium (Ca). In its elemental form, the calcium atom itself is an alkali earth metal that is relatively unstable, thanks to two valence electrons.1 Those electrons are easily lost, which leaves the calcium cation (Ca2+). The calcium cation has a positive valence because there are two more protons than electrons. For simplicity's sake, we'll just refer to Ca2+ as the calcium ion.2
To become a more stable compound, Ca2+ binds to things that have two or more valence electrons (2-) such as carbonate (CO32-), silica (SiO32-), sulfate (SO42-), or phosphate (PO43-). When these compounds oversaturate water, they form various forms of scale.
The most readily available among these is the carbonate ion, and calcium binds to it easily:
Ca2+ + CO32- → CaCO3
Calcium + carbonate → Calcium carbonate
In swimming pools with cementitious surfaces (like plaster, quartz, or pebble), carbonation also occurs from the interaction of a cement compound called calcium hydroxide (Ca(OH)2).3
Ca(OH)2 + CO2 → CaCO3 + H2O
Calcium hydroxide + carbon dioxide → calcium carbonate + water
Based on the evidence we have collected and lab tested, we believe the calcite crystals are created by the carbonation of calcium hydroxide as the water pulls it from the cement in the pool surface. See the video above.
Calcium carbonate saturation in water

Like anything else in nature, water craves equilibrium. The equilibrium it cares most about is the saturation of calcium carbonate. The international standard for measuring this saturation is the Langelier Saturation Index (LSI). If you have read our website and used the Orenda app, you know that we teach the critical importance of the LSI. It is the first of our Four Pillars.
0.00 on the LSI is perfect equilibrium. Below -0.30 is considered aggressive because water does not have enough CaCO3 in saturation...so the water will dissolve calcium to pull it into solution and get back to equilibrium. On the other end of the spectrum, if the LSI is over +0.30, water has too much CaCO3 in the given conditions, and it will get rid of some of it to bring itself back into equilibrium.
The LSI, however, only tells us the saturation of calcium carbonate, not other calcium compounds like calcium sulfate, calcium silica, or calcium phosphate. Keep that in mind as you read further in this article.
Calcite crystals vs. calcium carbonate scale
As we just mentioned, over the past several years we have collected samples of crystals from various pool owners and service companies from around the country. The crystals we are referring to in this article are formed during the winter in cold, stagnant water that is low on the LSI. These calcite crystals are not to be confused with calcium sulfate crystals, which are technically a type of scale that we cover in depth in another article.
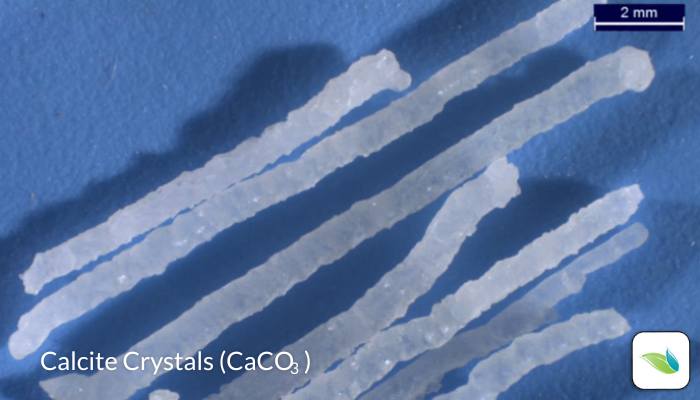
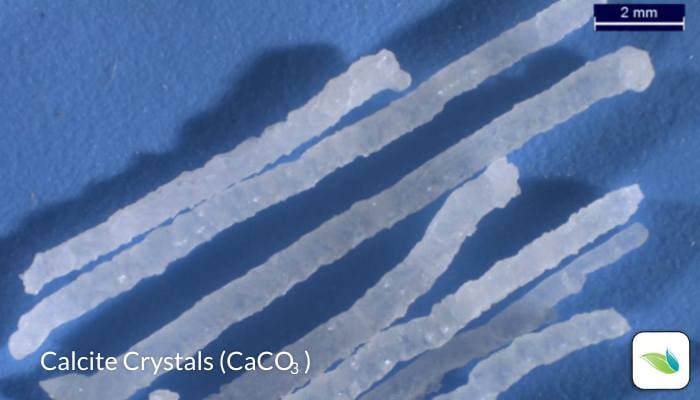
What are calcite crystals?
Thanks to lab tests and research, we now know quite a bit about these crystals. When we originally published this article in 2018, we did not yet know much about them. Here are some things we have learned:
- Calcium Crystals are a consequence of a severely low LSI, usually in cold, stagnant water over many weeks or months (winter, usually).
- We have no evidence that suggests crystals are the result of–or even related to–flawed plaster materials. We believe they are caused by unbalanced water chemistry during the winter.
- Crystals are not unique to any one brand or type of surface. Any cementitious surface (pebble, quartz, plaster, etc.) can be susceptible to calcite crystals.
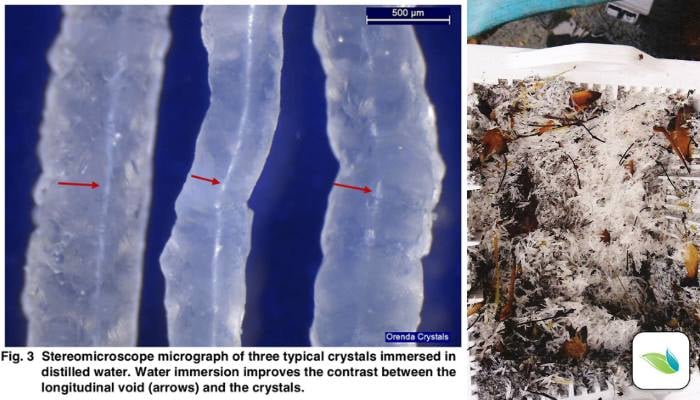
- We have abundant evidence that suggests crystals grow out of a surface due to water having a severe need for calcium saturation. In other words, the water is starving for calcium and extracts it from the plaster. We believe the high pH of calcium hydroxide creates a localized formation of calcite, much like a volcano or calcium nodule. This theory is backed by the fact that each crystal has a microscopic hollow void (or tunnel) in it. The scientists at the lab told us this indicates materials are being drawn out through the tunnel and deposited at the end, further growing the crystal.
- We do not yet know why some pools have winter dust and others have crystals. Our theory so far is that the type of pool cover matters. Solid covers are more prone to winter dust, whereas mesh covers are more prone to crystals.
- We know of at least four (4) distinct types of calcite crystals. We also know of other crystals that are not made of calcite, such as calcium sulfate scale, which is unrelated to this phenomenon.
- All forms of winter crystals that we know of can be prevented by having balanced-LSI water during the coldest days of winter. This means having enough calcium hardness and carbonate alkalinity in the water, allowing the pH to naturally rise up to its ceiling, and keeping it there.
Related: How to winterize a swimming pool
What is calcium carbonate scale?
We cover calcium carbonate scale more in-depth in its own article. Here is Orenda's definition of scale:
Scale is a buildup of mineral compounds (usually calcium-based) that have precipitated out of solution and have formed on surfaces in and around the water system.

There are several types of scale, as mentioned earlier. This is because calcium can bind with other ions with two or more electrons. Carbonate (CO32-) is the most abundant in swimming pools, therefore calcium carbonate is the most common form of scale. The LSI tells us how saturated water is with calcium carbonate, so an LSI value over +0.30 is scale-forming. It means the water is oversaturated and needs to get rid of some CaCO3 to get back to equilibrium.
The key here is that scale precipitates from the water and adheres to surfaces. If it's actually scale, the calcium compound–whatever it is–will stick to plastic fittings, lights, rails, and the face of tile. So if it did not originate from the water and land on surfaces, it is not scale.
But if the calcification is only on cement but not the non-cementitious surfaces, it cannot be scale. It is likely still calcium carbonate, but it originated from within the cement itself. Therefore it is not scale.
Many forms of calcification are often mistakenly referred to as scale. Calcite crystals are one such condition. There are also uneven carbonation/mottling issues on plaster, which are even more common than crystals. Just because something reacts and disappears in the presence of acid does not mean it's actually scale. It just means it was likely calcium carbonate. The origin of the calcium carbonate is what matters here. Did the calcium compound come from an oversaturation in the water, or not?
How to distinguish scale from calcite crystals
Calcium Carbonate Scale
- Scale precipitates from the water as a result of the water being over-saturated with calcium carbonate (over +0.30 LSI).
- Always forms in the highest-LSI areas first. Usually that means the warmest water first. Look for scale on salt cells, heat exchangers, spas, spillways, sunny spots in the pool, shallow water, and dark tile lines that absorb heat.
- Scale can form on non-cement surfaces. Calcium crystals cannot.
- Scale can also appear above the water line due to the 'wet/dry effect', where the water splashes on there but dries, leaving behind scale. Calcium crystals cannot.
- Acid can remove it, albeit aggressively. SC-1000 removes it more gently, by dissolving the calcium and chelating it to keep it in solution.
Calcium Crystals
- Occurs when water is under-saturated with calcium (below -0.30 LSI)
- Normally occurs over the winter in cold, stagnant water, when most etching damage is done to pools (again, low LSI).
- Will only appear on cement surfaces and tile grout...but never on the face of tile or other non-cement surfaces like plastic, glass, metal, etc.
- Only form below the water line because the water extracts calcium out from the plaster.
- Acid products can remove them in the short term, but the problem can come back again unless the LSI is balanced. If not, the water will still crave calcium, leading to more crystals the next winter.
- Common in pools that winterize with a normal or lower pH (< 7.4), and below 400 ppm calcium hardness.
Four types of calcite crystals found in swimming pools
Since 2017, we have collected many samples of crystals and have had them lab tested. As of now we know of at least four types of calcite crystals. They are all calcium carbonate, but they contain trace amounts of other elements like strontium, silicate, and a few others. We honestly still don't know why or how.
Of the four types of calcite crystals, we estimate just two of them account for more than 90% of the crystals we see and hear about.
1. The first we have nicknamed sandpaper crystals:

Sandpaper crystals are stubborn and sharp. These small translucent crystals feel like sandpaper and are notoriously difficult to remove. They tend to be resistant even to acid washes...despite being calcium carbonate (calcite). The oddest thing is they can be scraped or sanded off, then they dissolve just fine in acid. But when they're still stuck to the wall, they are not easily dissolved. It's still a mystery.
2. The second type we have nicknamed needle crystals:
Needle crystals grow from the cement into long and brittle formations. They have a hollow tube in the center of them and the lab called their molecular structure as "skeletal calcite". Compared to sandpaper crystals, these are relatively easy to chemically soften and remove. SC-1000, LSI balance (usually accomplished by raising calcium hardness) and brushing is generally sufficient to soften and remove these as the water warms up over 65ºF.
3. The third type, which is much less common than the first two, we have nicknamed toenail crystals:
.jpg?width=700&height=400&name=Calcite%20crystals%20underwater%20(2).jpg)
Toenail crystals look like...well... toenails. They are usually off-white in color and a bit more opaque than the translucent needle and sandpaper crystals. Toenail crystals are sharp and we have seen them as long as half an inch. They are without a doubt the ugliest of the four crystals. But don't tell them we said that. The good news is, toenail crystals seem to be the easiest crystals to chemically dissolve and remove. Simply balancing the LSI and getting SC-1000 in the water when it's above 65ºF and rising seems to do the trick. Brushing speeds things up too. We have heard of these crystals simply vanishing in a week or two, almost like they were never there. It's wild.
4. Finally, the rarest of the four types of calcite crystals we have nicknamed flaky crystals:
Flaky crystals look like snowflakes or pieces of broken translucent glass. They tend to populate in certain areas of the pool, as opposed to uniformly everywhere like the other three types of crystals. Our theory is these flaky crystals only grow where the cement is most porous and weak. But we don't have enough data to know for sure. We do know they are rare and thankfully they are easy to remove, similar to toenail crystals. They seem to be a bit more stubborn, but relative to sandpaper crystals, flaky crystals are easy to remove.
Treatment: do's and don'ts
Calcium Carbonate Scale
- DO test your pool water (and tap water!) for calcium hardness, alkalinity, and pH. Log your results, either on the Orenda App and email them, or by hand.
- DO adjust your pH and alkalinity to balance your LSI. The Orenda Calculator™ can help.
- If you use cal hypo, DO consult a service professional about potentially switching to a different type of chlorine. It may not be necessary, but never make a switch without a trained professional.
- DO use SC-1000 or sequestering agent to help dissolve the calcium scale back into solution. For scale above the water line, raise the water line so the treated water can soak it.
- DON'T use acid products if you're not a trained pool professional, with the proper safety gear. Acid products are okay to use for scale removal, but be aware of their impact on the pH and the LSI.
- DON'T treat for scale without being sure it's actually scale.
Calcite Crystals
- DO test your pool water (and tap water!) for calcium hardness, alkalinity, and pH. Log your results, either on the Orenda App and email them, or by hand.
- DO adjust your pH and alkalinity to balance your LSI. The Orenda Calculator™ can help.
- ADD CALCIUM. The Orenda Calculator™ will tell you exactly how much calcium chloride you need to add. If the pool is one that gets winterized, we recommend 400+ ppm calcium hardness. For pools with mesh covers, you also need to take future dilution from rain and snow into consideration. For those pools, we recommend winterizing with over 500 ppm calcium hardness.
- DO use SC-1000 to help dissolve the calcium crystals back into solution and remove them from your walls.
- DON'T use acidic products as a strategy, because your problem is with a low LSI (your plaster is bleeding out calcium). Adding acid will make the problem worse in the long term, despite maybe having short-term results.
- DON'T drain the pool and acid wash. The better strategy is to chelate calcium while the pool is full, chemically break down the calcium crystals into solution, and go from there. If you insist on draining your pool, do it after the calcium crystals are gone.
- DON'T expect SC-1000 to remove crystals overnight. It can take several weeks, especially in colder water temperatures.
Conclusion
In either case–calcium carbonate scale or calcium crystals–the LSI is your measurement of success. If your LSI is balanced, neither problem can occur in the first place. Preventative care, anyone?
Beware of the trap: acid products work before your eyes against both problems. That said, if acid is to be used at all, it should only be on carbonate scale, not calcium crystals. In the case of calcium crystals, get the pool's calcium level up (we say 300-400 ppm) to balance the LSI and stop the bleeding, then use something like SC-1000 to chemically dissolve the crystals back into solution. You cannot reverse the damage already done, but you can prevent further damage from occurring.
If you are in a climate requiring winterization of pools, management of the LSI becomes even more critical, because colder temperature yields a lower LSI. Play around with our calculator and watch how the LSI changes in real-time.
For more questions or general advice on how to manage these problems, we are available to speak with you in confidence. Just contact us. Thank you for taking the time to read all this. If you know someone who would find this article valuable, please share it with them.
1 CK-12 Textbook. (2022). 6.10: Alkaline Earth Metals. Introductory Chemistry (CK-12). UC Davis LibreTexts.
2 Calcium ion is technically a cation because its protons outnumber its electrons. We will be referring to it as a calcium ion to reduce confusion. We just wanted to mention this technical detail just in case you're a chemist with free time on your hands who wants to call us and tell us we're wrong. And in all seriousness, if we're wrong about something please do contact us! We want to make sure everything we publish is accurate.
Source: Parikh, M., Webb, S. (2012). Cations: Potassium, Calcium, and Magnesium. Continuing Education in Anaesthesia Critical Care & Pain, Volume 12,(4), pp. 195–198. DOI: 10.1093/bjaceaccp/mks020
3 Vance, Falzone, Pignatelli, et.al. (2015). Direct Carbonation of Ca(OH)2 using Liquid and Supercritical CO2: Implications for Carbon-Neutral Cementation. Industrial & Engineering Chemistry Research. 54 (36) 8908-8918. DOI: 10.1021/acs.iecr.5b02356

