The Orenda Startup® is about LSI balance, not just Calcium

Many people think The Orenda Startup® is a calcium-based startup. But that's not always the case, and we want to set the record straight. The Orenda Startup® is an LSI-based startup.
Covered in this article:
- The Langelier Saturation Index (LSI)
- Calcium hardness is just one factor in the LSI
- How does this relate to swimming pool startups?
- Calcium hardness is just one factor in the LSI
- The Orenda Startup® is not the first, nor the only LSI-based startup
- The goal of an LSI-based startup
- Why even bother with an LSI startup?
- Problems that can occur with the Orenda Startup®
- Undershooting the LSI target
- Overshooting the LSI target
- Both problems look identical
- Types of plaster application problems
- Conclusion
The Langelier Saturation Index (LSI)
First, a quick recap of the Langelier Saturation Index (LSI). The LSI is an index that measures the saturation equilibrium of calcium carbonate (CaCO3), where 0.00 is considered perfect balance.
Too much calcium carbonate in the given chemistry conditions means an oversaturation, and the LSI value would exceed +0.30. Too little calcium carbonate in the given chemistry conditions means an undersaturation, and water is hungry for it. This means aggressive, etching water with an LSI below -0.30.
There are six chemistry factors that must be accounted for when calculating the LSI.1 They are:
- Water temperature
- pH
- Carbonate Alkalinity
- Calcium Hardness
- Cyanuric Acid (CYA)
- Total Dissolved Solids (TDS)
For more information about the LSI, we cover it in many other articles on our website and in our help center. You can search using the search icon at the top of our website or in the footer below. It's so important that LSI balance is our First Pillar of Proactive Pool Care.
While all six (6) LSI factors matter, calcium hardness stands out as one of the most important, especially during new plaster startups.
Calcium hardness is just one factor in the LSI
Water is governed by nature and physics. It seeks only one thing: equilibrium. We measure that equilibrium using the LSI. In fact, LSI balance is the only thing water craves. And that balance is based on the saturation of calcium carbonate.
There's an important difference between the amount of calcium in the water and how saturated the water is with it. The amount is measured as calcium hardness (ppm or mg/L). The LSI tells us how saturated the water is with that calcium.
How does this relate to swimming pool startups?
Cementitious pool finishes like plaster, quartz, and pebble contain calcium compounds in them. One of those compounds––calcium hydroxide (Ca(OH)2)––becomes carbonated during the cement curing process. But when fresh water is introduced to the pool, this still-curing cement can interact with the water if the water is not LSI-balanced.
While we have countless ways to balance the LSI using the Orenda Calculator™, water has only two ways to balance itself: eat or scale.
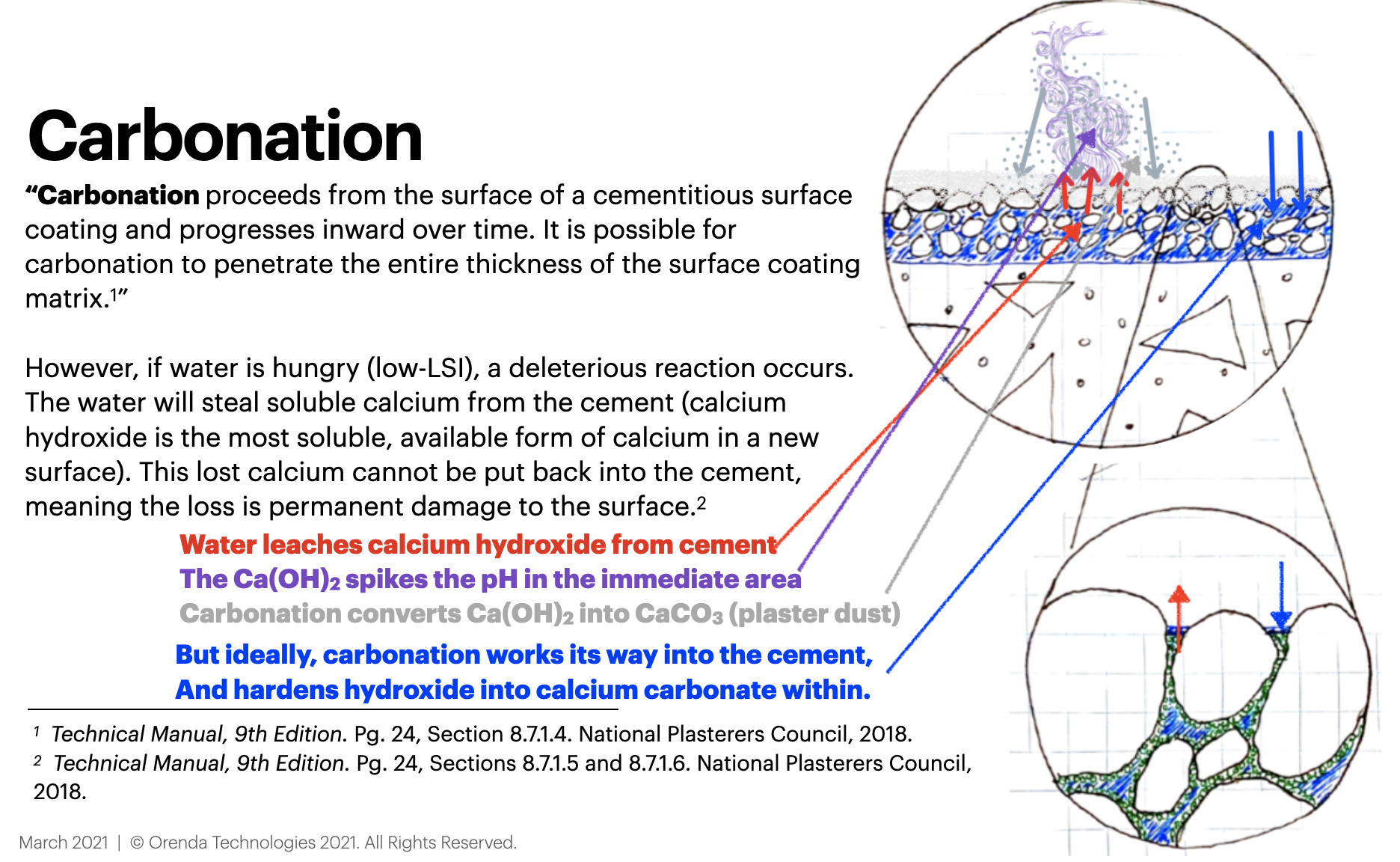
The main concept is this: low-LSI water is hungry for calcium, and it will dissolve it into solution from anywhere it can find it. The path of least resistance is not-yet-cured calcium hydroxide from the cement. This leads to plaster dust formation and a spiked pH. Traditional startup methods do not prevent this problem from occurring; on the contrary, they virtually guarantee it.
The research and our own field experience show that etching virgin plaster (and its subsequent consequences of plaster dust and a spiking pH) can be avoided altogether. All you need to do is fill the pool with slightly-positive LSI water. And that's what the Orenda Startup™ does.
The Orenda Startup® is not the first, nor the only LSI-based startup
Filling a pool with slightly positive-LSI water (+0.20 and above) was not originally an Orenda idea. We assume many pool builders, pool service pros and plaster applicators have tried treating water as it fills a pool to try to avoid problems. Once such company is Blue Moon Pools out of Santee, California. Lupe Mariscal from Blue Moon gave us the idea.2
So while companies may have been trying to do it, it was difficult without being able to chelate calcium early on. Sequestering agents are acidic and may not be able to get the job done. But SC-1000 enables early addition of calcium while filling the pool. Over the years, the process has evolved into a proven procedure with tens of thousands of success stories across North America.
But we were not the first startup that focused on the LSI instead of range chemistry.
The earliest startup method we know of is "The Bicarb Startup" from the research group onBalance. Like ours, the Bicarb Startup method was forged out of necessity. Both methods share the same goal: fill the pool with slightly-positive LSI water so the plaster can cure properly without water stealing calcium from it.
The Bicarb Startup, as its name suggests, leans heavily on sodium bicarbonate to raise the alkalinity of the fill water. Indeed, high alkalinity accelerates the curing process of cement. Our process usually leans on calcium chloride, but it depends on the tap water chemistry. If the tap water has high calcium already, but low total alkalinity, we advise our customers to do the bicarb startup.
Do what is needed to get the water slightly LSI-positive while it's filling the pool. It's as simple as that.
The goal of an LSI-based startup
We just mentioned that both the Orenda Startup® and the Bicarb Startup fill the pool with slightly-positive LSI water. But why not perfectly LSI-balanced?
Technically speaking, 0.00 LSI water can still dissolve calcium hydroxide, which is still in the process of being converted into calcium carbonate. The LSI is about calcium carbonate saturation. So 0.00 LSI will not dissolve CaCO3, but it can still dissolve Ca(OH)2. For this reason, we need an LSI value that is slightly higher than we would want during normal pool operations.
OnBalance's research states that the LSI needs to be around +0.50 to prevent the loss of calcium hydroxide. And while that may be true, our field experience has shown +0.20 to +0.29 is sufficient to prevent plaster dust. Our startup procedure indicates you can go up to +0.49 LSI if necessary. In either case, to prevent plaster dust, the water needs to be above 0.00 LSI.
We used to advocate for specific minimums of calcium and alkalinity...but as this process has evolved, we have learned to just focus on the LSI instead.
We do not require (nor encourage) always adding calcium on a startup. The chemistry of the fill water determines everything. So let's do an example using the Orenda Calculator®.
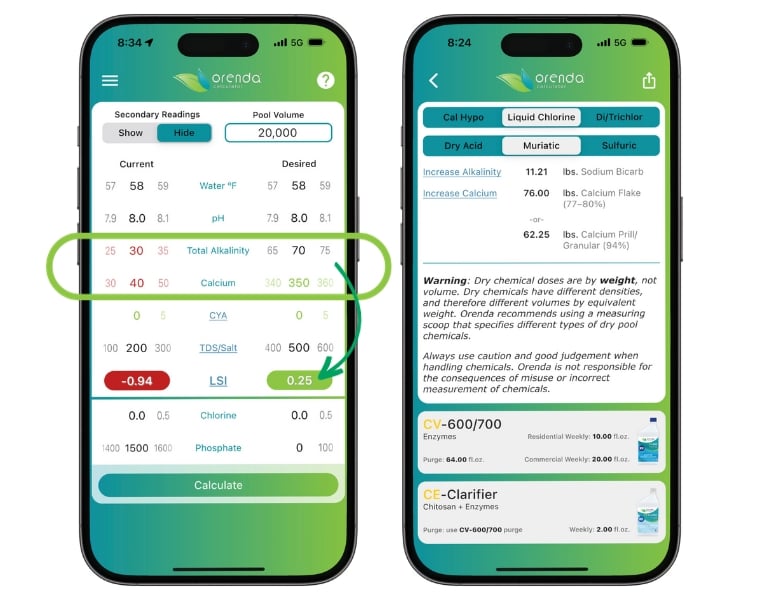
Example: The fill water is 58ºF, with 8.0 pH, 30 ppm TA, 40 ppm CH, no CYA, and 200 ppm TDS. The LSI of the fill water is -0.94 (very aggressive).
The Orenda Startup® wants +0.20 to +0.29 LSI. We accomplish this by increasing both TA and CH (dissolved and added separately). Calcium hardness is the larger priority in this scenario, so on fill-up day we would add 76.00 pounds of calcium flake, or 62.25 pounds of calcium prill. The next day (or several hours later) we would add 11.21 pounds of sodium bicarbonate to increase TA.
If done correctly, an LSI-based startup process should prevent water from taking calcium hydroxide from the cement. This means no plaster dust and no spiking pH for the first several days after fill-up.
Why even bother with an LSI startup?
You may be thinking this is a lot of extra work, and unnecessary. But first consider this: every speck of plaster dust that comes out of the surface is carbonated calcium that should have cured within the cement.
You can hide it all you want with startup products like SC-1000 or other sequestering agents. You can also burn up the dust with a hot start, but we strongly advise against hot starts.3 But neither of those methods address the real problem. You cannot put calcium back into the surface after it has been taken.
We know from the research done by the National Plasterers Council, the American Concrete Institute, and the Portland Cement Association that the cement curing process takes time. During the curing time, plaster is vulnerable. Calcium Hydroxide (Ca(OH)2) remains soluble until it carbonates into calcium carbonate (CaCO3), which reinforces the strength of the cement...and that curing process takes months. It continues on indefinitely for many years.
We also know that slightly-positive LSI removes the water's ability to leech out calcium from a new surface. Water is incapable of over-saturating itself with calcium. So if we prevent the water from stealing calcium hydroxide from the cement while the plaster is most vulnerable, that's a good thing.
So why bother prioritizing the LSI during startup? Because the LSI is what matters to water. Either we give the water the balance it craves, or the water must balance itself...at the expense of the brand new plaster finish. That's not fair to the homeowner, nor is it fair to anyone else involved.
Problems that can occur with the Orenda Startup®

When you decide to do an LSI-based startup like ours, the sequence of operations is critically important. Going out of order on our step-by-step startup procedure will likely mess up the results. Failure to follow the instructions is not a failure of the procedure itself. We have had questions and frustrations with our process, only to find out that only part of the process was followed.
Even when the process if followed faithfully, things can still go wrong by accident. Usually it's either overshooting or undershooting the target LSI.
Undershooting the LSI target
When you miss the target LSI on the low side (meaning not enough calcium or alkalinity, or not fast enough), the pool's water will likely have a spiked pH and some plaster dust. There should still be less plaster dust than normal, because even if you miss the mark, you're still adding calcium or sodium bicarb to get closer to the LSI than the normal tap water alone.
The pool still needs to be LSI balanced, but you're looking at water that already balanced itself by eating some calcium hydroxide. The pH is probably too high to just add calcium to the water, because over 8.2 pH, calcium has difficulty dissolving at all. So we must bring down the pH while simultaneously adding pre-dissolved, chelated calcium. To do this, follow our Post-Fill Startup procedure and apply your tested chemistry values in the Orenda Calculator™ to find your correct chemical dosing.
We know this is not perfect, but it is effective at stopping the issue and allowing you to regain control of the chemistry. It's the best we can do, given the circumstances.
Overshooting the LSI target
 If too much calcium is added to the water (or it's added too quickly), it may not all stay in solution. Another way to overshoot the LSI is if you start adding calcium too late, like when the pool has been filling for a few hours already. This can happen because by that time, the pH has probably already spiked due to the water dissolving calcium hydroxide in the first few hours of fill-up. This is not the same as plaster dust, but it is still calcium carbonate. We call this "snow", or "snowing".
If too much calcium is added to the water (or it's added too quickly), it may not all stay in solution. Another way to overshoot the LSI is if you start adding calcium too late, like when the pool has been filling for a few hours already. This can happen because by that time, the pH has probably already spiked due to the water dissolving calcium hydroxide in the first few hours of fill-up. This is not the same as plaster dust, but it is still calcium carbonate. We call this "snow", or "snowing".
This is one of the many reasons why it is critical to be there prior to the water turning on. We need to start from the beginning.
We had a customer do a startup with a faulty test kit. Their test that showed 120 ppm calcium in the source water...but in reality, the source water had 300 ppm calcium. Our procedure would have said to adjust alkalinity first...but instead, calcium chloride was added, thinking there was only 120 ppm filling the pool. The result was a lot of snow that kept coming back, day after day.
That said, the pH never spiked, which indicates the dust was not from calcium hydroxide loss.
Orenda Startup® Failures look almost identical
Both overshooting and undershooting the LSI leads to dust, which uniformly falls onto the surface. High calcium hardness or high LSI does not have the ability to create unique marks and blemishes like straight lines, angles, or foot prints. It never has, and never will.
Actual plaster dust (low LSI) can come from within any cement, including walls and corners of steps. Snowing (high LSI) will not do this because it originates from the water itself, and therefore gravity brings the dust only onto the floor and other horizontal surfaces. You will not see dust on the walls if you overshot the LSI.
One final point here: a proper LSI-based startup does not hide problems caused by bad plaster application or exposure.
Types of plaster application problems
Any blemish or flaw left behind by the plaster application will likely be visible when doing the Orenda or Bicarb startup. Why? Because we are removing the water's ability to pull any calcium from the surface.
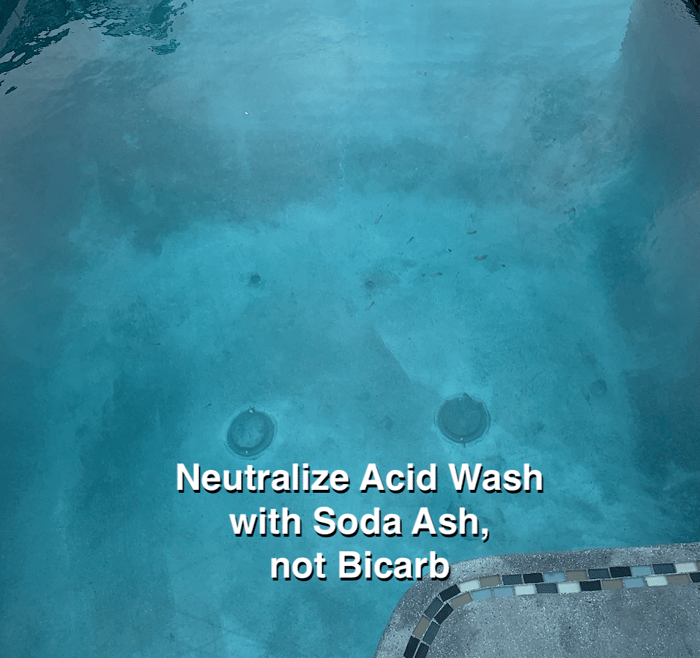
If there are puddle marks, streaks, lines, footprints, rings, motting and other physical marks, you will probably see them. These imperfections will not be burned off, like they would during a hot start. We recognize this is can be seen as a burden and disadvantage for builders and applicators, because we all want the pool to look great. Everybody does...especially the customer. When the exposure is done right, everybody is happy. When it's done wrong, nobody is happy.
Our startup is about preventing the loss of calcium so the cement in the plaster can cure properly. It neither creates nor conceals visible flaws from application or acid exposure. Here are some of the most common issues we see that have nothing to do with startup water chemistry. These are pre-existing conditions left behind by plaster application:
.jpg?width=1200&length=1200&name=bad%20acid%20wash%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=very%20bad%20acid%20wash%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=wet%20troweling%20lines%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=clinker%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=dribble%20marks%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=footprints%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=puddle%20marks%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=streaking%20water%20and%20acid%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=weepers%20(plaster%20issues).jpg)
.jpg?width=1200&length=1200&name=too%20many%20issues%20to%20name%20(plaster%20issues).jpg)
Again, a method like a hot start would burn off the outermost layer of the plaster surface, and many of these problems would be removed...but you would also be removing valuable calcium hydroxide from the surface, which compromises it long-term. With an LSI startup like ours or the Bicarb startup, these marks stay.
Not only does water in the pool not cause these problems, our startup physically cannot cause these problems (nor remove them). We are open about the benefits AND limitations of our products and procedures. If you have marks like the ones shown above, that's a separate issue that needs to be handled.
Conclusion
Proper startups are not meant to fix bad application issues. They are meant to balance the water so the water does not steal calcium from the cement.
Too often, issues like the ones described in this article are blamed on improper water chemistry. And while there are plenty of ways bad water chemistry can cause problems, it's not fair to blame water chemistry for everything. We hope this article clarifies expectations of what an LSI startup can and cannot do.
1 If borates are used in the pool, they are a seventh factor. The Orenda Calculator™ factors in borates if you use them.
2 Thank you Lupe!
3 A zero-alkalinity process (aka acid bath or hot start) should not be considered a startup at all. It is an exposure method that came about to burn off the top layer of plaster. This can temporarily make the pool surface look more uniform, but if done too early (i.e. in the first 30 days), whatever blemishes and flaws existed from the original application and exposure can come back. Hot starts are about the worst thing we can do to fresh cement. It is much better to wait at least 30 days before doing a low-alkalinity treatment (acid bath), because the cement will at least be more cured and ready to have some mottling removed.
