How Swimming Pool Enzymes Work

Enzymes are proteins and amino acids that catalyze certain chemical reactions. Swimming pool enzymes like CV-600 are made to break down and remove non-living organics and oils, which helps reduce chlorine demand and clarify the water.
Covered in this article:
- Oxidation vs. Enzyme breakdown
- What enzymes do
- Oxidation pros and cons
- Enzyme pros and cons
Oxidation vs. Enzyme Breakdown
Oxidation is a chemical process that destroys a substance. Technically, oxidation is when electrons are stolen from an oxidant (like bather waste or metals), permanently changing it. In swimming pools, oxidation is basically burning contamination out of the water.
The primary oxidizer in swimming pools is usually the primary sanitizer, chlorine. Your pool may be using alternative sanitizers like bromine too. Some swimming pools utilize secondary oxidation/disinfection systems like Ozone and AOP, which provide a second layer of oxidation of anything that passes through their area of effect.
Enzymes catalyze a specific type of chemical reaction, and there are countless variations of enzymes. In our bodies alone there are thousands of enzymes that break down and digest food and convert it into various nutrients that can be processed by our bodies.
Swimming pool enzymes are designed to break down carbon-chain non-living organic material. They convert carbon bonds into carbon dioxide (CO2), which off-gasses from the water. What's left over is much easier for chlorine to oxidize. So in effect, enzymes lower the energy level required for chlorine to destroy complex non-living organics and oils.
What enzymes do
Enzymes work by attracting (or seeking out) a given type of molecule, known as a substrate. The enzyme has a special place that fits that type of substrate and nothing else, and either binds two substrates together or breaks them apart. Swimming pool enzymes like CV-600 and CV-700 break the non-living organics apart, metabolize the carbon bonds, and convert them into CO2. This CO2 then off-gasses as small clusters of bubbles on the pool surface, which is noticeable for several hours after the initial enzyme treatment called the purge.
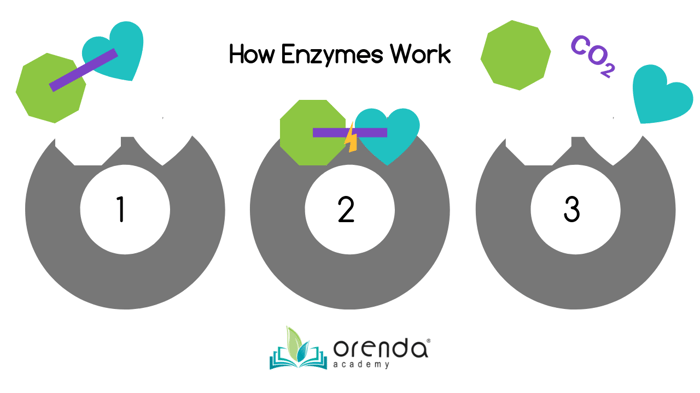
Above is our renaissance-quality illustration above. In step 1, an substrate (non-living organic oxidant) is drawn to the enzyme, which has a perfectly suited space for it to land in. Step 2 shows the carbon bond is broken when lodged in the enzyme's grasp. Finally, the separated components are released along with CO2.
Here is a very quick video demonstrating the other way enzymes work:
In swimming pools, the enzymes we use are called broad-spectrum enzymes, because they look for carbon-chain substances to break down.
Oxidation pros and cons
Oxidation destroys substances, but does so at the expense of the oxidizer, because along with oxidation comes reduction. The electrons stolen from the oxidant have a negative charge (e-), which reduces the charge of the oxidizer. Eventually, that oxidizer has no more ability to steal electrons, and can therefore no longer oxidize. Chlorine, for instance, gets reduced to inert chloride ions (Cl-).
When we speak about chlorine being "used up", that means it was reduced. Learn more about this in our article about Oxidation-Reduction Potential (ORP). And while chlorine is an excellent sanitizer, it is a relatively weak oxidizer...especially compared to powerful oxidizers like ozone or hydroxyl radicals. A benefit of oxidation is it can destroy a wide range of substances, and there are several types of oxidizers available to use in swimming pools.
A downside to oxidation is that it may not completely eliminate contaminants. Look no further than the tile line of the swimming pool. If you have a scum line on there, chlorine is falling short of fully removing the oils and organics that created it. In some cases, chlorine is incapable of finishing the job.
Perhaps the biggest downside to oxidation is the byproducts left behind. This is especially true for complex nitrogen compounds, which yield disinfection byproducts (DBPs) like chloramines.
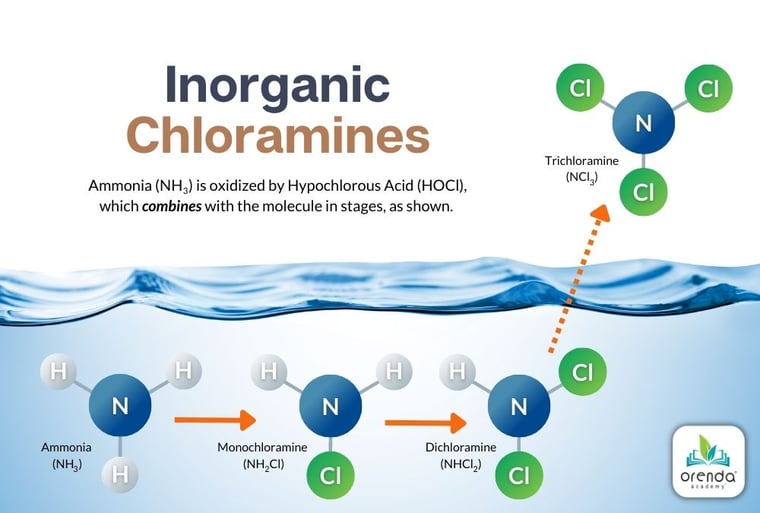
These byproducts are harmful to people and are also leading contributors to corrosion in indoor swimming pool facilities. There needs to be a better way of handling non-living organics so that we are not solely relying on chlorine. And that's where enzymes come in.
Enzyme pros and cons
Enzymes are excellent at simplifying complex, non-living organic substances in water. Organics may include decaying leaves and grass clippings from around the pool, or natural body oils, mucus, and sweat. These substances are major contributors to the oxidant demand (and therefore the chlorine demand).
And yet, natural organics are relatively easy for chlorine to oxidize when compared to synthetic organic materials like bather products. Think deodorants, soaps, perfumes, body lotions, cosmetics, tanning oils, and sunscreen. These substances are not easy for chlorine to oxidize.
Enzymes, however, easily break these substances down into their most basic ingredients. By removing the carbon bonds, even the most complex organic substance (think something like sunscreen) becomes much easier for chlorine to fully destroy.
There are some oxidants that enzymes cannot address, like metals and ammonia, or other nitrogen compounds. That being said, if you have organic nitrogen compounds like urea, enzymes can break down the carbon bonds and simplify it into inorganic ammonia and other byproducts.
Without enzymes, the process of breaking down urea takes a lot more chlorine, which yields more disinfection byproducts throughout the process. And remember, chlorine is being reduced the entire time.
If chlorine oxidation were as effective as enzymes are against bather waste, pools relying on chlorine only would not have problems like scum lines and oily water. They would rarely struggle with cloudy water. And their filters would not get fouled by grease and grime. But the busier the pool, the more obvious it is that chlorine alone falls short. And that's why supplementing chlorine is the action step of our second pillar of proactive pool care.
Conclusion
One key to optimal water quality is minimizing the sanitizer demand. Since most pools use chlorine of some kind, we'll refer to this as the chlorine demand. And remember that chlorine's primary purpose in water is disinfection.
Thankfully, only a small percentage of chlorine demand is germs, which chlorine should be able to quickly and easily kill. If you have algae, it can have a more noticeable impact on chlorine demand. Everything else is the oxidant demand:
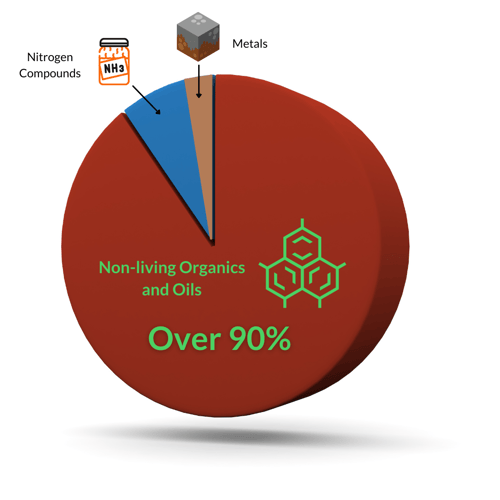 Of the oxidant demand, over 90% of it can be addressed by enzymes. So why not simplify water chemistry by removing non-living organics from the water? Why not let enzymes do the heavy lifting against bather waste, and let chlorine get back to what it does best?
Of the oxidant demand, over 90% of it can be addressed by enzymes. So why not simplify water chemistry by removing non-living organics from the water? Why not let enzymes do the heavy lifting against bather waste, and let chlorine get back to what it does best?

