What Causes High Alkalinity in Pools?

Have you ever managed a swimming pool where the alkalinity level fluctuates? Usually, alkalinity decreases each week due to acid use, but what about an alkalinity level that rises? Those of you with CO2 feeders are probably already familiar with a seemingly endless rise in alkalinity. If you have ever wondered why alkalinity sometimes climbs on its own, this article is for you.
Covered in this article:
- What is alkalinity?
- How to raise alkalinity in a pool
- Why CO2 feed systems increase alkalinity
- Hypochlorite byproducts
- Nitrogen compounds
- Conclusion
What is alkalinity?
We have several articles in the Orenda blog about alkalinity already, so we'll hyperlink to them here, then give a quick summary:
Total Alkalinity (TA) is the sum of dissolved alkali in the water; specifically, substances that can take Hydrogen ions (H+). The ability to take Hydrogen slows the drop in pH, hence alkalinity is a measure of the pH buffering capacity of water that resists a drop in pH. The opposite of alkalinity would be acidity, which resists the rise in pH. In some respect, while it contributes to TA, boric acid serves the opposite (mirror) function of alkalinity, in that it resists a rise in pH by taking hydroxides (OH-) instead of Hydrogen (H+).
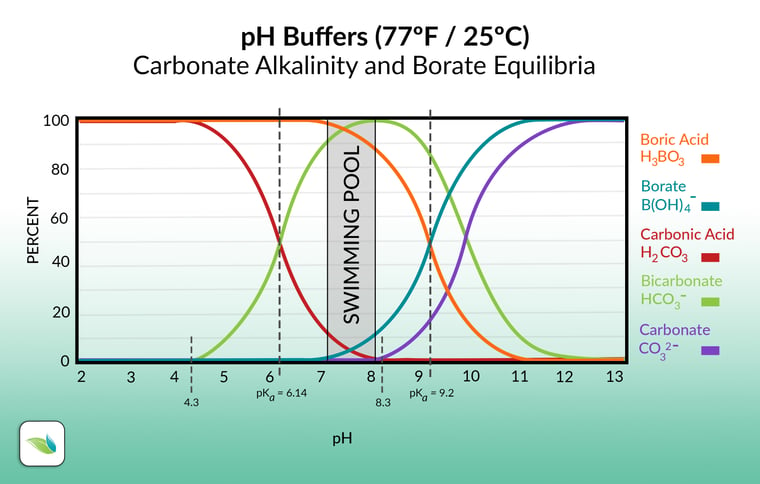
Most alkalinity in swimming pools will be in the carbonate alkalinity category, which we will discuss shortly. At usual pool pH, the carbonate alkalinity is composed mostly of the bicarbonate ion (HCO3-); the same bicarbonate as found in sodium bicarb (baking soda).
The rest of the total alkalinity is comprised of any other substances that help to buffer the pH from changing too rapidly. Foremost among these other substances is Cyanuric Acid (CYA), which creates cyanurate alkalinity. Borates can also contribute to total alkalinity, though they are less significant than CYA. Alkalinity also includes Hydroxides (OH-), which usually contribute at small levels, but as you'll learn in this article, add up in certain situations.
Carbonate alkalinity, or "corrected alkalinity"
When we say "carbonate alkalinity", we are referring to any and all alkalinity containing carbon dioxide in it, as shown in the chart below. This chart excludes other alkalinity types that contribute to total alkalinity (TA), such as cyanurate alkalinity and borate. This exclusion is also known as "correcting" alkalinity, as is required for an accurate LSI calculation.
Now let's look at just the carbonate alkalinity without borates:
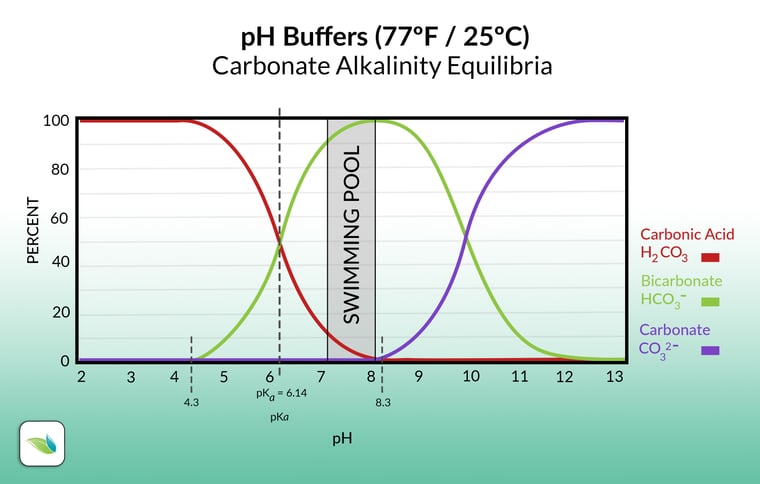
When carbon dioxide dissolves in water, it is in equilibrium with carbonic acid (H2CO3), illustrated by the red line. Anything under 4.3 pH is carbonic acid. Everything in the chart above contains carbon dioxide in some form or another. Hence the term "carbonate alkalinity". As the pH rises, carbonic acid dissociates and converts into bicarbonate (HCO3-) and a free Hydrogen ion (H+). Further pH rise over about 8.3 makes bicarbonate convert into carbonate (CO32-) by losing another Hydrogen ion, so, starting from carbonic acid, you now have carbonate and two free Hydrogen ions. This may sound confusing but it will make sense later in this article.
Now let's get to the meat and potatoes of today's subject: what causes high alkalinity in pools? The first way should be obvious: you can raise alkalinity yourself.
How to raise alkalinity in a pool
Increasing alkalinity is as easy as adding more carbonates to the water. The safest, most cost-effective, and popular product is sodium bicarbonate (aka baking soda, or bicarb). Sodium bicarb is great for raising alkalinity, and also slightly raises the pH of the water, because its pH is only about 8.3. Looking back at the alkalinity chart above, you will see that most alkalinity in pool chemistry is bicarbonate. The other product to raise alkalinity is sodium carbonate (aka soda ash), but this is better for raising pH strongly and raising alkalinity slightly. Soda ash has a much higher pH of 11.3 (in a 1% solution), making it about 1000 times more basic than sodium bicarb.
Related: How to add sodium bicarb or soda ash to a swimming pool
To raise alkalinity with sodium bicarb, you can figure out your dose using a dosing chart or the free Orenda App (free on the App Store and GooglePlay). Once you know the amount of sodium bicarb needed, pre-dissolve it in a bucket of water and add slowly around the perimeter of the pool. Here's the procedure:
And while sodium bicarb is the most common way to raise alkalinity, pools with CO2 feeders tend to see alkalinity rise on its own.
CO2 feed systems increase alkalinity
 Why do pools using CO2 for pH control often struggle with constantly rising alkalinity? To explain this phenomenon, we want to first share this presentation by Richard Falk. Technically speaking, injecting CO2 does not directly impact alkalinity. But anyone with a pool on a CO2 system already knows, the pool's alkalinity increases over time without adding sodium bicarb or soda ash. This is a verified fact.
Why do pools using CO2 for pH control often struggle with constantly rising alkalinity? To explain this phenomenon, we want to first share this presentation by Richard Falk. Technically speaking, injecting CO2 does not directly impact alkalinity. But anyone with a pool on a CO2 system already knows, the pool's alkalinity increases over time without adding sodium bicarb or soda ash. This is a verified fact.
To explain why refer back to the alkalinity chart earlier once again. It's worth asking the question another way: how does CO2 lower pH in the first place? We covered this in our article about CO2 and Henry's Law already, but we'll refresh your memory. The more CO2 dissolved in your water, the lower the pH, and vice versa. So injecting CO2 directly into the water lowers the pH, because most of it becomes carbonic acid, and carbonic acid brings the pH down. Simple enough.
For a while now, we have had a solid theory–assumed as true because it made so much sense–that alkalinity rises because additional CO2 eventually converts into more bicarbonate alkalinity than the pool had before. True enough, except that the alkalinity chart above only shows us part of the equilibria...the carbonates themselves. The chart does not show where the additional Hydrogens go. Here are the complete equilibria:
CO2 + H2O ⇌ H2CO3 ⇌ HCO3- + H+ ⇌ CO3-- + 2H+
carbon dioxide + water ⇌ carbonic acid ⇌ bicarbonate + Hydrogen ⇌ carbonate + 2 Hydrogen
These free Hydrogen ions (H+) are very important! They perfectly negate the additional bicarbonate created by injecting CO2, so from an alkalinity standpoint, it's a wash. So that theory is only half-true at best.1 Thanks to our guru chemist friend Richard Falk, we now understand exactly why alkalinity rises in CO2 pools. And it happens for two reasons.
Hypochlorite byproducts
Alkalinity rises because of excess hydroxide in hypochlorite chlorines. And in the case of calcium hypochlorite (cal hypo), there is not just excess hydroxide, there is excess carbonate too.2 This excess hydroxide (and in the case of cal hypo, excess carbonate) contributes to alkalinity, and therefore the alkalinity rises. This, however, is only a slight increase in alkalinity because when chlorine oxidizes or kills, HOCl (strong, killing form of chlorine, hypochlorous acid) becomes HCl (hydrochloric acid, aka muriatic acid) and brings the pH and alkalinity mostly back down. Either way, this is still a net increase in alkalinity without having to add sodium bicarb or anything else.
Furthermore, these hydroxides raise TA in pools that use acid and sodium bicarb for pH and alkalinity control too, but a little extra acid negates this, so nobody notices.
Nitrogen compounds
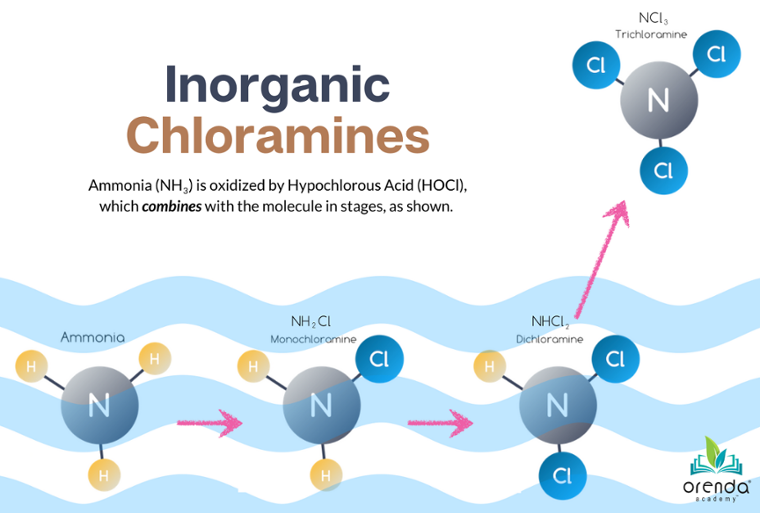 Expanding upon the previous point about hydroxides from hypochlorite chlorine, the alkalinity can rise faster if chlorine oxidation does not complete its process. Incomplete oxidation, let's call it, means less HCl created to bring the pH and alkalinity back down. This can occur when chlorine is combined with nitrogen compounds like urea and ammonia.3 You know this as combined chlorine.
Expanding upon the previous point about hydroxides from hypochlorite chlorine, the alkalinity can rise faster if chlorine oxidation does not complete its process. Incomplete oxidation, let's call it, means less HCl created to bring the pH and alkalinity back down. This can occur when chlorine is combined with nitrogen compounds like urea and ammonia.3 You know this as combined chlorine.
Related: Understanding Breakpoint Chlorination
Conclusion
We thought the inevitable rise of alkalinity in pools with CO2 feeders was due to additional carbonic acid being introduced, which eventually converted into more bicarbonate than the water had before. This theory not only made sense to us, but it also made sense to everyone we spoke to about this while showing the alkalinity chart. That is until we asked an actual chemist. As it turns out, alkalinity rises due to excess hydroxides left behind by hypochlorite chlorines: sodium hypochlorite (liquid chlorine) and calcium hypochlorite (cal hypo).
There is a minor net rise in TA when all things are fully oxidized in the water. But there is a more rapid rise in TA when chlorine does not fully oxidize things, and instead combines with nitrogen compounds like urea and ammonia. This negates the production of hydrochloric acid (HCl) from the chlorine that would otherwise bring the pH and alkalinity down after oxidation. Instead, alkalinity climbs and climbs.
This was a dense subject to cover, and we hope we simplified it enough so it can be understood. If you have questions, please comment below. Thanks for your time.
1 This is a real bummer too because our theory made so much sense when looking at the alkalinity chart. The chart only shows alkalinity, however, and deliberately excludes Hydrogen ions. This is why we fact-check everything we publish here at Orenda. We want to make absolutely sure our information is correct. We're glad we can set the record straight on this.
2 68% Cal hypo chlorine creates free chlorine (HOCl ⇌ OCl-) but leaves behind byproducts. So 68% of the product becomes chlorine, and the other 32% in typical product consists of 18% chloride salt, 1.5% calcium chlorate (Ca(ClO3)2), 0.5% calcium chloride (CaCl2), 1.0% calcium hydroxide (Ca(OH)2), 2.5% calcium carbonate (CaCO3), and 8.5% water (H2O). Source: Richard Falk, and ScienceDirect.
3 Thanks again to Richard Falk for explaining this chemistry to us. For the sake of simplicity, we are not including all of the chemistry involved in this, but we may publish a PDF someday with all of the equations. To us, it makes sense, as heavier used pools notice a higher rate of alkalinity increase than lightly used pools. This ties back into the broader conversation about chloramines and why they are so important to get under control.
