Understanding Cyanuric Acid (CYA)

Cyanuric Acid (CYA), also called stabilizer or conditioner, protects chlorine from sunlight. But CYA is a double-edged sword, causing a significant impact on both water quality and water balance. CYA is so important to pool chemistry that we decided to make Minimal CYA our fourth Pillar of Proactive Pool Care.
Covered in this article:
- What is Cyanuric Acid (CYA)?
- CYA and sunlight
- CYA, alkalinity and LSI
- How does cyanuric acid work?
- How to increase cyanuric acid in a pool
- How to lower cyanuric acid in a pool
- Draining and diluting
- Downsides of cyanuric acid
- CYA decreases chlorine speed
- CYA decreases ORP
- CYA increases acid doses
- CYA decreases LSI
- Conclusion
What is Cyanuric Acid (CYA)?

Cyanuric acid (CYA), also known as stabilizer or conditioner, is widely used in the pool business. It serves as a protection shield for chlorine against sunlight degradation (called photodecomposition or photolysis).1,2 It is also a pH buffering system that contributes to Total Alkalinity (TA).3 This article explains both of these functions.
Stabilized pools are fundamentally different than non-stabilized pools. While CYA is in some ways a miracle product, too much of it creates problems like over-stabilization. Therefore, understanding CYA is a cornerstone of what we teach at Orenda.4 Let's get into it.
CYA and sunlight
The Sun’s ultraviolet rays degrade non-stabilized chlorine quickly, creating a problem for outdoor pools. Studies show that sunlight can wipe out chlorine by 75-90% in a matter of two hours.1,2,3 This makes it impossible to hold chlorine for 7 days (a standard weekly professional pool route) without automated chlorine feeding or saltwater chlorine production.
With cyanuric acid, however, chlorine can last much longer. It is not permanently protected from UV degradation, but even low levels of CYA make a major difference in chlorine staying power.5
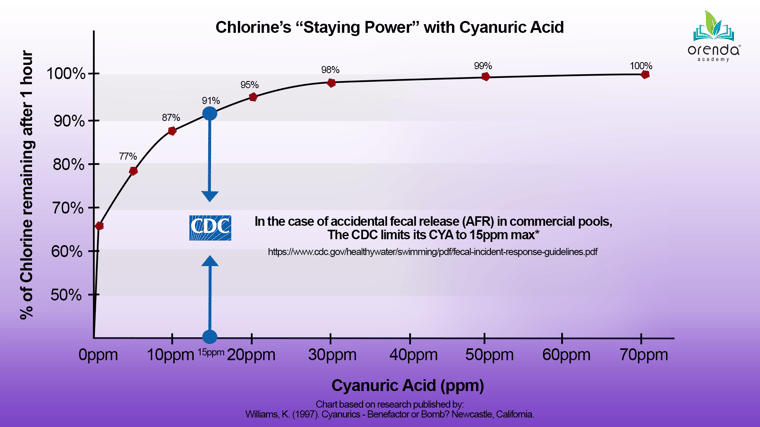
We will elaborate more on this graphic later in this article. For now, notice how much sunlight protection is provided by low levels of CYA. Higher levels of CYA have a diminishing return on sunlight protection benefits.
CYA, alkalinity, and the LSI
CYA also impacts water balance. Cyanuric acid and its conjugate base, cyanurate ion, are a pH buffering system. This buffering system is called cyanurate alkalinity. We have talked about this at length in our Rule Your Pool Podcast:
Here's a graphic showing cyanurate alkalinity overlayed with carbonate alkalinity:

The more CYA in the water, the more acid is needed to reduce the pH in a swimming pool. Approximately one-third of CYA ppm is cyanurate alkalinity. This matters because of the impact on overall water balance, measured using the Langelier Saturation Index (LSI).
Cyanurate alkalinity must be subtracted from Total Alkalinity (TA) to find the carbonate alkalinity, which is is needed for the LSI formula. Thankfully, the Orenda Calculator® does all of these corrections for you automatically. You can see for yourself in the calculator.
Orenda Calculator > SHOW secondary readings > increase and decrease CYA, and look at how the carbonate alkalinity number fluctuates, while the TA does not.
This difference between TA and carbonate alkalinity is cyanurate alkalinity.6
How does cyanuric acid work?

The cyanuric acid molecule is a hexagon with alternating Nitrogen and Carbon atoms. It allows for three molecules of chlorine to attach to the nitrogen, forming a weak nitrogen-chlorine bond (N-Cl). Because the N-Cl bond is weak, it allows for chlorine to let go of CYA when it has something to oxidize or kill. When attached to CYA, however, chlorine is protected from sunlight. Cyanuric acid is kind of like sunscreen for chlorine. This compound is called a chlorinated isocyanurate.
Any type of chlorine added to water will dissolve and create the active form, Hypochlorous Acid (HOCl), and its slower and weaker conjugate base, Hypochlorite ion (OCl-). HOCl will attach to CYA's nitrogen bond until it has a contaminant to oxidize or kill. It then detaches and does its job, and another chlorine will take its place.
We know the Nitrogen-chlorine (N-Cl) bond is weak because the chlorine attached still shows up in a free chlorine test. If the bond were stronger—like that of chloramines and other disinfectant byproducts—chlorine would only show up on a total chlorine test, not free chlorine.
A metaphor: Imagine a floating raft that chlorine holds onto. When it needs to leave the raft to oxidize or kill a germ, chlorine simply lets go of the raft…and another chlorine molecule will take it’s place and grab the raft. As long as chlorine is holding onto the raft, it’s protected from sunlight. When it lets go, it’s active free available chlorine, but vulnerable to sunlight.

How to increase cyanuric acid in a pool
Pure cyanuric acid is available as a granular solid and as a liquid. Most commonly, however, cyanuric acid is used in two stabilized chlorine products:
- Dichlor (sodium dichloro-s-triazinetrione dihydrate)
- Trichlor (trichloro-s-triazinetrione)
These stabilized chlorines have about 50-58% CYA (by weight), which means they are primarily CYA products. We elaborate on chlorine product percentages in this article. Just because a product (like trichlor) says 99%, that does not mean it's 99% chlorine. It means it's 99% trichlor.
In our experience, it is these stabilized chlorine products that cause overstabilization. Pools that are treated with granular or liquid cyanuric acid do not have a constantly-rising CYA level each week, making responsible CYA levels is easier to maintain.
We have these figures in the Orenda Calculator®, but we also want to use them here to illustrate some example doses of CYA, and how it climbs quickly. These figures are all based on 10,000 gallons of water:
| Product | Dose | CYA impact | Cl impact |
| Cyanuric Acid (granular) | 1 pound | ↑ 11.98 ppm | 0 |
| Trichlor (tabs) | 1 pound (two 3" tabs) | ↑ 6.68 ppm | 11 ppm |
| Sodium dichlor (granular) | 1 pound | ↑ 6.4 ppm | 4.4 ppm |
As you can see, one pound of stabilized chlorine puts more than 6 ppm of CYA in 10,000 gallons of water. It adds up quickly.
Recommended cyanuric acid levels in a pool
There seem to be as many different opinions and recommendations about CYA levels as there are sources. Everyone has an opinion, and so do we. For commercial/public pools, we advise following the CDC's recommended limit of 15 ppm. This is important for public health, especially in larger bodies of water.
For residential pools, we agree with Robert Lowry's recommendation of 30-50 ppm CYA, or simply less than 50 ppm. Minimal CYA is our Fourth Pillar of Proactive Pool Care.
How to lower cyanuric acid in a pool
There are several ways and experimental methods of reducing cyanuric acid in swimming pools.
Oxidation over time
Over several months, cyanuric acid can break down and be oxidized by chlorine. The exact rate of decomposition depends on the molar ratios of each chemical and factors like water temperature and sunlight... but generally speaking, this is a very slow process. Cyanuric acid will break down into organic nitrogen compounds similar to urea. Chlorine will then combine with it as part of the oxidation process, creating combined chlorine.
Eventually, when breakpoint chlorination is achieved, these byproducts will be destroyed and the CYA is gone. It takes a lot of time and free chlorine for reduce CYA this way, though for most swimming pools, some of this happens automatically from normal pool maintenance and chlorination.
Nitrifying bacteria
There are also products on the market designed to accelerate this process by breaking down CYA, which can reduce the demand on chlorine to finish the job. From what we are told, these products are a type of nitrifying bacteria that require dechlorination in the pool before use. We have heard these products deliver mixed results, but we are optimistic such technology can improve over time.
Specialized cyanuric acid removal filters
We also have optimism that specialized filters will one day be able to pull CYA out of the water efficiently. This would help pool owners and operators maintain minimal CYA levels. As of now, some filters do exist, but again, they deliver mixed results. We have not met any customers that have found them reliable yet.
Alum flocculant
The method of using alum to floc out CYA (aluminum cyanurate) is still being developed, but it shows some promise. You can learn more about it here.
Reverse Osmosis (RO) filtration
One proven way to reduce CYA in pools is to filter it using reverse osmosis (RO). RO systems can be parked near the pool, and pump water from the pool through the system and back into the pool. RO filters remove just about everything from the water, including TDS, calcium, salts, alkalinity, disinfection byproducts, metals, and more. It can be expensive, but in places where droughts are a concern, water restrictions may make RO the only viable option.
We think RO filtration is a great solution to give pools a fresh restart, but it should be noted that the filtered water will have almost no calcium or alkalinity in it when the process is finished. That means balancing the LSI is an urgent priority when the filtering is completed, otherwise the filtered water will seek calcium from anywhere it can find it.
Draining and diluting
By far and away, the most cost-effective and reliable way to remove CYA from the water is to remove water and replace it. Partial draining and diluting also reduces other parameters like Total Dissolved Solids (TDS) and calcium hardness. These items tend to accumulate as water evaporates and fresh water replaces it.
Dilution is different from evaporation loss, in that evaporation only removes distilled water, leaving everything else behind.
Related: When to dilute your swimming pool
It should be noted that cementitious pool finishes (like plaster, pebble, quartz) tend to hold some CYA even when the pool is drained. We have many customers who have completely drained over-stabilized swimming pools, only to refill and test over 50 ppm CYA with fresh water. The theory is that the CYA absorbs into the cement, but we have not seen conclusive studies on this.7
Even if this is the case, draining and diluting remains the most cost-effective cyanuric acid reducer.
The downsides of cyanuric acid
While some CYA provides the major benefit of protecting chlorine from UV degradation, too much of it leads to problems. Let's briefly touch on the drawbacks of CYA, and why overstabilization is such a problem in swimming pools.
CYA decreases chlorine speed
The main issue with CYA is that it drastically slows chlorine down. The more CYA, the slower the chlorine, and it is not a linear relationship. Higher CYA means longer contact times (CT values) to kill harmful pathogens in the water. There is so much peer-reviewed research on CYA's impact on chlorine's killing efficacy that we cannot even link to all of it here.8 We encourage you to research it for yourself.
The concept of chlorine strength is somewhat of a misnomer because it's really about the concentration of HOCl and time. So when you hear things like "a lower pH makes chlorine stronger", that's referring to a non-stabilized pool having a higher percentage of HOCl relative to its slower and weaker counterpart, OCl-. See the left chart below.

As you can see on the left, as the pH rises, the percentage of Hypochlorous acid (HOCl) drops, as its Hydrogen leaves, and we are left with Hypochlorite ion (OCl-). Hence, higher pH yields weaker, slower chlorine.
But that's only in a pool without cyanuric acid.
With just 30 ppm CYA, look for the red line (HOCl) on the chart on the right. Notice how flat and low the curve is? That's because most of the free chlorine in the water is bound to CYA, creating chloroisocyanurates, as represented by the purple line in the chart.
How does pH impact chlorine in a stabilized pool?
The chart above represents how little the pH impacts the chlorine efficacy (%HOCl). The difference in %HOCl between 7.0 and 8.5 pH is hardly noticeable. We would say negligible. At 7.5 pH, approximately 97-98% of the free chlorine in the water is bound to CYA, leaving maybe 3% unbound. Of that 3% unbound chlorine, the pH will impact how much of that chlorine is HOCl vs. OCl-.

Free chlorine to CYA ratio: 7.5% of CYA minimum
Based on HOCl calculations and years of empirical evidence from thousands of pools, the figure of 7.5% has been floating around in the swimming pool industry.9 While this figure has not been conclusively proven, it seems to work well in outdoor pools and is a decent target to aim for.
Let’s put this formula into the real world. If you have 100 ppm CYA, your new minimum to stay ahead of algae growth is approximately 7.5 ppm chlorine. Can you sustain that?
If you had only 50 ppm CYA, that drops to 3.75 ppm chlorine, which is more attainable, especially on a weekly visit.
CYA decreases ORP
Increasing cyanuric acid lowers Oxidation Reduction Potential (ORP). ORP probes measure the conductivity specific to redox reactions, expressed in millivolts (mV). ORP is a good proxy to measure in real-time alongside free chlorine readings and pH.
If the free chlorine and pH levels are kept the same (which requires chemical automation, which is still not perfect), ORP is a good way to determine the efficacy of chlorine. Increasing chlorine will increase ORP because it increases the amount of HOCl, while decreasing pH will also increase ORP because it increases the percentage of HOCl.
Cyanuric acid distorts this, because it severely lowers the percentage of HOCl, as shown in the chart in the last section. That said, even with CYA in the water, ORP is still highly sensitive to pH, due to the movement of Hydrogen ions (H+) affecting the electrical charge of different substances in water. The Hydrogen ions and HOCl are on the same side of the reaction below, so increasing either one will increase ORP, and vice versa.
HOCl + H+ + 2(e-) ⇄ Cl- + H2O
Hypochlorous Acid + Hydrogen ion + 2 electrons ⇄ Chloride ion + water
CYA increases acid doses
Remember that cyanurate alkalinity is a pH buffer. Refer to the chart mentioned earlier. The pKa of cyanurate is 6.88, which is closer to pool chemistry than that of bicarbonate (6.27). This means by molar weight, cyanurate is a stronger pH buffer than bicarbonate...but on a ppm basis, bicarbonate is still the dominant buffer.
Because CYA is a pH buffer, Increased CYA levels also increase the amount of acid required to do a given pH reduction. You can see this for yourself using the Orenda Calculator®. Plug in a given pH reduction at a consistent TA on both sides of the calculator. Then increase the CYA and see how it increases the acid dose required to lower the pH.
This translates to more acid needed, which translates to more costs.
CYA decreases the LSI
Continuing along this same thread about cyanurate alkalinity, it needs to be subtracted from TA when calculating the LSI. The more CYA, the higher the proportion of TA is cyanurate. The LSI, however, only wants the carbonate alkalinity. Again, you can see this for yourself using the Orenda Calculator®. Increase the CYA and you will see the LSI decrease, along with the carbonate alkalinity. This will also decrease the pH ceiling (because the carbonate alkalinity is being decreased).
How to calculate cyanurate alkalinity
While the Orenda Calculator® calculates cyanurate alkalinity in the background automatically, it's helpful to know how it is calculated. The exact math is precise, but the simplified math can be done pretty easily, even if it's not as precise. The general rule of thumb is one-third of the CYA level.
But 1/3 is only an estimate. The pH also impacts this slightly. See the chart below:
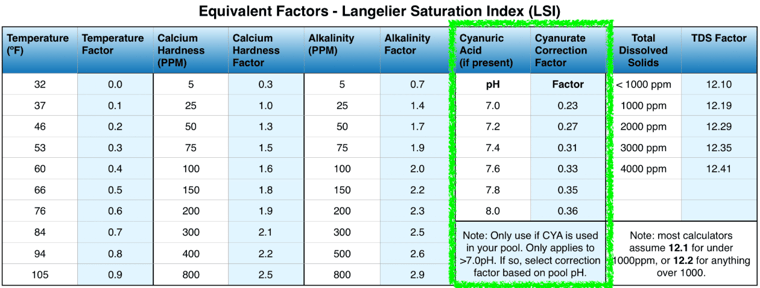
As you can see, it's only 1/3 if the pH happens to be 7.6. If the pH is 7.2, the multiplier drops to 0.27. Let's do an example of 60 ppm CYA at 7.8 pH.
Cyanurate Alkalinity = (CYA) x (CYA correction factor @ current pH)
Cyanurate Alkalinity = (60) x (0.35)
Cyanurate Alkalinity = 21 ppm
To calculate the carbonate alkalinity (aka corrected alkalinity), simply subtract the cyanurate from total alkalinity.6
TA - cyanurate alkalinity = carbonate alkalinity
For pools using trichlor or dichlor regularly, CYA climbs quickly. It is not uncommon to see pools with over 200 ppm CYA. For easy math, let's say the pool has 210 ppm CYA (assuming the person testing took the time to properly dilute the sample with distilled water per the test kit instructions). One third of 210 is 70 ppm cyanurate alkalinity. If your TA is 80 ppm, that means you have only 10 ppm carbonate alkalinity, which would likely lead to aggressive water (low LSI). This is not uncommon, unfortunately.
Conclusion
To quote the late Robert Lowry: Cyanuric acid protects chlorine from sunlight, and it controls your pool.10 CYA impacts both water quality and water balance. As you can see from the depth of this article, it is a multi-faceted issue that complicates water chemistry, yet is necessary for outdoor pools to have any hope of holding chlorine for more than a few hours.
Stabilization is not the problem...overstabilization is. Avoid overstabilization and it will be much easier to maintain a clean and healthy pool.
Our recommendation is to keep CYA to a minimum (our Fourth Pillar), so that you can still have decent chlorine speed while also benefitting from sunlight protection. Do not forget that more CYA means more acid required for pH corrections, and a lower LSI. We hope this in-depth article was helpful for you to better understand cyanuric acid.
1 US Environmental Protection Agency. (1992). Chlorinated Isocyanurates. R.E.D. Facts, US EPA Office of Prevention, Pesticides and Toxic Substances. EPA-738-F-92-010.
2 Nowell, Lisa N., Hoigné, Jürg. (1992). Photolysis of aqueous chlorine at sunlight and ultraviolet wavelengths––I. Degradation rates. Water Research. Vol. 26 (5), pp. 5993-598.
3 Wojtowicz, John. (2004). Effect of Cyanuric Acid on Swimming Pool Maintenance. Journal of the Swimming Pool and Spa Industry. Vol. 5 (1), pp. 15-19.
4 There are too many studies to showcase in this article, but we do link to many of them in our other articles about cyanuric acid.
5 Williams, K.M. (2000). Cyanurics ~ Benefactor or bomb?
6 If borates are used in the water, borates also must be subtracted from TA to find carbonate alkalinity. So the difference between TA and carbonate in that case might be cyanurate alkalinity + borate alkalinity. The Orenda Calculator® does this correction automatically if borates are enabled in the app settings.
7 If you want to conduct such studies, it would be a huge benefit to the industry! Contact us and let us know how we can help.
8 You can start with these four studies. There are dozens more...
Falk, R.A.; Blatchley, E.R., III; Kuechler, T.C.; Meyer, E.M.; Pickens, S.R.; Suppes, L.M. (2019). Assessing the Impact of Cyanuric Acid on Bather’s Risk of Gastrointestinal Illness at Swimming Pools. Water. (11), 1314.
Canelli E. (1974). Chemical, bacteriological, and toxicological properties of cyanuric acid and chlorinated isocyanurates as applied to swimming pool disinfection: a review. American journal of public health, 64(2), 155–162.
Shields, J.M., Arrowood, M.J., Hill, V.R., & Beach, M.J. (2009). The effect of cyanuric acid on the disinfection rate of Cryptosporidium parvum in 20-ppm free chlorine. Journal of water and health, 7 1, 109-14.
Chen, Z., Su, Y., Chen, J., Li, Z., & Wang, T. (2024). Study on the health risk of cyanuric acid in swimming pool water and its prevention and control measures. Frontiers in Public Health, 11.
9 The 7.5% figure originated from work done by Ben Powell and Richard Falk, based on data from John Wojtowicz, Dr. Stanley Pickens, Dr. Ellen Meyer, and a few others. It was published online in various places, but became mainstream when Robert Lowry published it in his books, and taught it in his classes. Nobody has claimed the figure is exactly precise, because there are many variables. But the concept is sound and it's a good rule of thumb. To prevent algae, you need at least 7.5% of whatever your CYA ppm is.
Years later, Robert Lowry theorized that using of 50 ppm borate would reduce the factor from 7.5% down to just 5%. Read his tech bulletin here.
Richard Falk estimates that keeping phosphate levels below 500 ppb could reduce the 7.5% as well, but an exact figure is unknown. It is estimated to be around 4 to 4.5%. To his credit, Richard has published that these theories have not been tested or proven. They are based on models and calculations. Still, he makes a good case for it, which is why we have adopted the goal of maintaining phosphate levels below 500 ppb as our Third Pillar of Proactive Pool Care.
10 Credit to the late Robert Lowry, who in 2016 published an amended version of his pool chemistry textbook, mainly to update the chapter about cyanuric acid.
Lowry, Robert W. (2016). IPSSA Basic Training Manual (2016 Revised Edition). Pg. 108.
