Understanding Henry's Law, CO2 and pH

This lesson explains why pH naturally rises. If you maintain pools on a weekly basis, you have probably noticed that the pH is almost always higher after a week. That's not your fault. It's natural. This article explains why.
Covered in this article:
- Carbon Dioxide determines the pH of water
- Lowering pH
- Raising pH
- Henry's Law of the Solubility of Gases
- How to contain pH (without fighting it)
- LSI-first approach
- Measure and dose acid correctly
- Conclusion
Carbon Dioxide determines the pH of water
First things first: technically speaking, the concentration of Hydrogen (H+) ions determines the pH. But in practice, there's something easier to conceptualize. Since we have carbonate alkalinity in our water as our primary buffering system, the Hydrogen ions are bound up in that buffering system. which means the amount of dissolved carbon dioxide (CO2) determines the pH of the water.
↑ dissolved CO2 = ↓ pH
↓ dissolved CO2 = ↑ pH
The most common source of acidity in water is dissolved CO2, so the more CO2 in the water, the lower the pH. This is because when CO2 dissolves in water, a small portion of it becomes carbonic acid (H2CO3). The reaction looks like this:
CO2(aq) + H2O ⇌ H2CO3(aq)
Carbon dioxide + Water ⇌ Carbonic Acid
Carbonic acid lowers the water's pH. So, how do we get more CO2 in the water to lower the pH? Let's consider how we lower the pH in a swimming pool.
Lowering pH
Chemically speaking, we can lower pH in a few ways by simply increasing the amount of carbonic acid in our water.
We can inject CO2 directly into the water, which reduces pH but does not reduce alkalinity.
Adding acid, on the other hand, lowers pH and alkalinity. For acid to create more carbonic acid, bicarbonate ions must be converted into carbonic acid by adding hydrogen to them.1 In the chart below, acid converts bicarbonate (HCO3-) into carbonic acid (H2CO3).
H+ + HCO3- ⇌ H2CO3
Hydrogen ion + bicarbonate ion ⇌ carbonic acid
When bicarbonate is converted into carbonic acid, total and carbonate alkalinity are reduced. CO2 injection, however, bypasses alkalinity and directly adds carbonic acid to the pool. This means CO2 injection does not reduce alkalinity.
As mentioned above, this still involves the concentration of Hydrogen ions, but it is how those Hydrogen ions fluctuate the percentage of carbonic acid vs. bicarbonate alkalinity. See the chart below. Adding acid adds an "H" to the green line, converting it into the red line, which moves the pH to the left (lowers the pH).
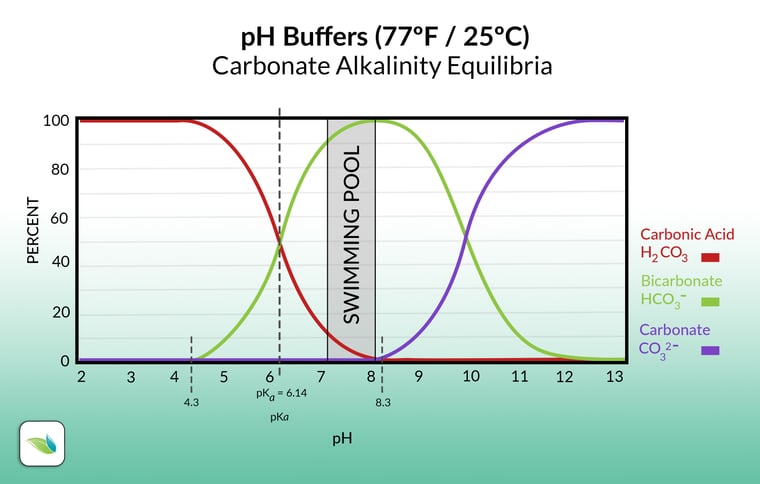
Raising pH
The opposite is also true. The pH will rise as CO2 is removed from water.
One way to do this is to add a pH adjuster like soda ash (sodium carbonate) or sodium bicarbonate.2 Another way to raise pH is to let it rise naturally via off-gassing CO2. There is natural aeration and accelerated aeration, such as spa jets, spillways, vanishing edges (infinity pools), and other water features.
Another source of aeration is salt chlorine generators. Most believe that salt systems raise the pH because of the high pH byproduct produced. Indeed, we taught this idea for many years (until we learned more). But it's actually the physics of aeration––which reduces the amount of CO2 in the water––that raises the pH in saltwater pools.
Any reduction of dissolved CO2 will increase the pH in water that contains carbonate alkalinity. Algae blooms fall into this category because algae consume CO2 and pull it out of solution. Therefore, the pH of a green pool tends to be very high. See the photo below of a digital pH probe testing an algae-filled green pool. It's pH was 10.39!

Algae aside, Henry's law of physics applies to the natural loss of CO2 via aeration.
Related: Total Alkalinity vs. pH and their roles in water chemistry
Henry's Law of the Solubility of Gases
Henry's Law is a law of physics formulated by William Henry in 1803. It states:
"At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid." An equivalent way of stating the law is that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid." (emphasis added) 3
Henry's Law basically states that the amount of a gas dissolved in water will strive to be directly proportional to the amount of that same gas in the air above the water.
As it pertains to swimming pools, we care most about CO2. With CO2, the atmosphere above a swimming pool has less CO2 in it than the carbonated pool (based on partial pressure). So CO2 leaves the pool over time, trying to equalize with the air.
The video below explains it well. Feel free to watch the entire video, but as it pertains to this article, we really only care about the first two minutes before he gives the soda example:
Once equilibrium is reached, off-gassing stops
The inverse of Henry's Law equation is also true. Gases leaving the water, once directly proportional to the air above the water, will stop off-gassing. This is because equilibrium has been reached. This is the same concept as a carbonated drink going flat.
We will explain why this is significant in the next section (or you can just take a shortcut to that section here). Yes, aeration can temporarily force CO2 out of the water, but thanks to Henry's Law, it would be pushed back into the water and redissolve. To cite our earlier source:
"Carbon dioxide enters the water through equilibrium with the atmosphere. CO2 (aq) << CO2 (g)"1
Because of Henry's Law, we know that pH has a natural limit. At a certain point, atmospheric pressure will push CO2 back into the pool. If the pH is going to rise above that natural limit, it has to be forced.
The pH ceiling (pH(eq))
Thanks to Henry's Law, we know that CO2 will off-gas until it reaches equilibrium with the air above the pool. That point of equilibrium is a limit, or ceiling. Since pH rises as CO2 leaves the water, we call this the pH ceiling of a swimming pool.
And yes, pH can absolutely rise above this ceiling, but not naturally. Something must force the pH above the natural pH ceiling, such as etching a calcium-rich plaster surface, which leeches a high pH into the water, or someone adding soda ash to a pool incorrectly.
The pH ceiling explains why almost all pools naturally have a rising pH each and every week. Carbon dioxide is naturally off-gassing, thanks to physics, so you're not doing anything wrong. The exact pH ceiling depends on your carbonate alkalinity (or corrected alkalinity) level:4,5,6
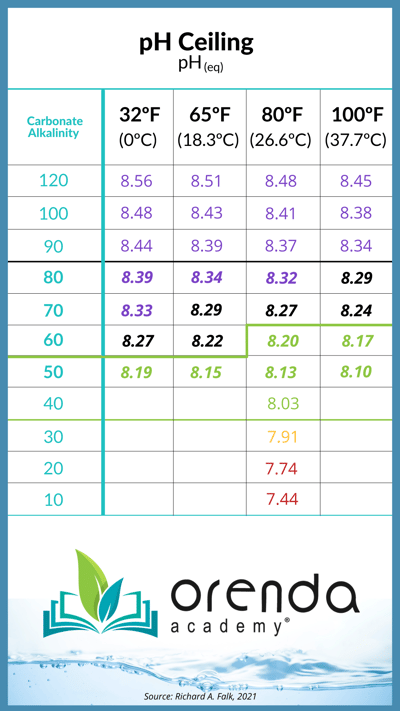
This chart is a general representation of the values for where water's pH will naturally rise based on the water temperature and carbonate alkalinity. To make it easier for our customers, we have also added pH ceiling and carbonate alkalinity as secondary readings in the Orenda Calculator™. You can see these numbers change in real time as you adjust various chemistry parameters.
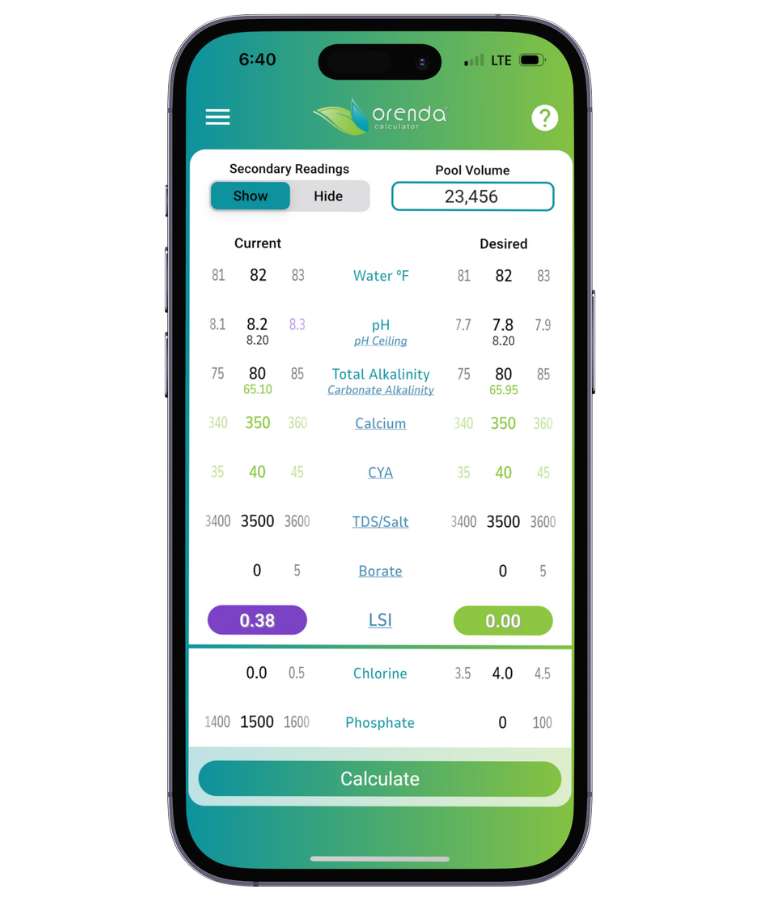
How to contain pH (without fighting it)
Ask any pool professional how easy it is to maintain a pH of 7.4- 7.6 for a week. Without chemical automation, a trichlor feeder, and/or an auto-cover on the pool, it's physically impossible.
Trying to control pH is futile. But thanks to Henry's Law, we can contain pH.
Related: Containing pH vs. Controlling pH
We can determine the carbonate alkalinity of our water, and therefore we can determine the pH ceiling too. That allows us to effectively limit how high the pH rises, which helps us maintain disinfection and water quality.7
LSI-first approach
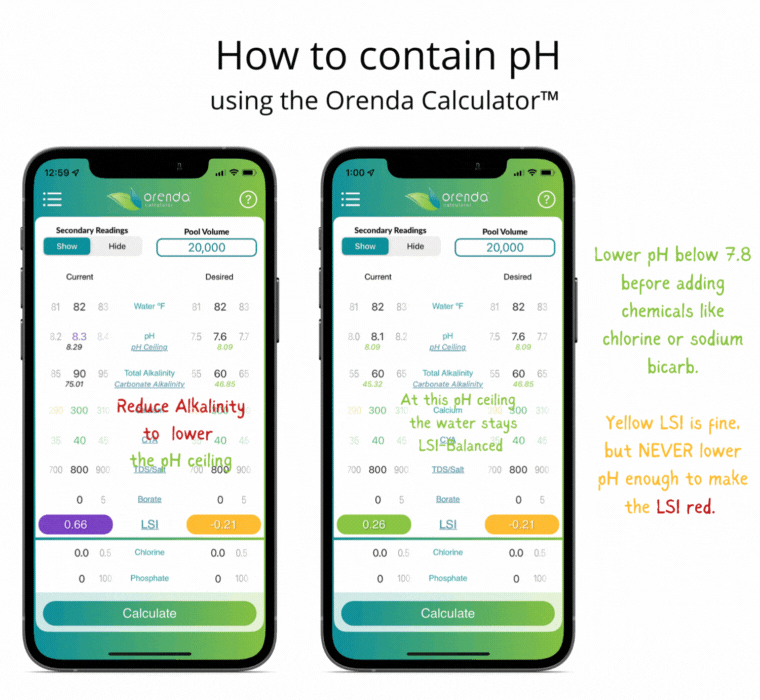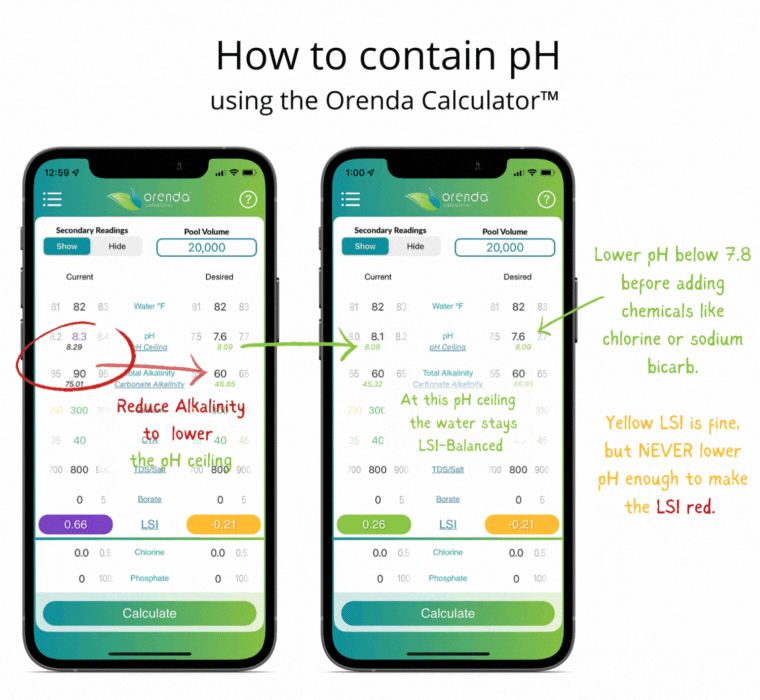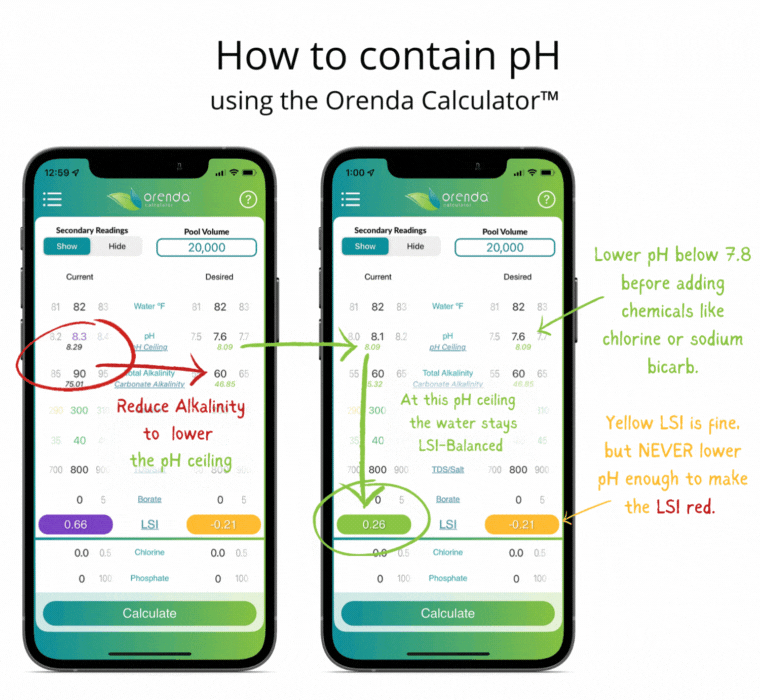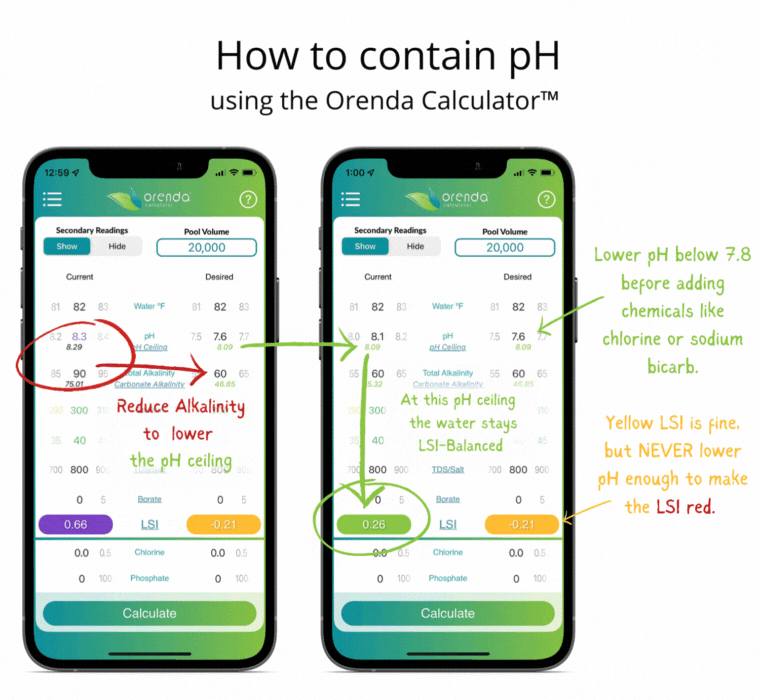
With all of this information in mind, here's how we recommend managing the physics at play.
- Set the calcium hardness level (based on temperature extremes) to allow for flexibility while maintaining LSI balance year-round (Pillar 1).
- Set the total alkalinity level (based on the primary sanitizer type) to limit the carbonate alkalinity and, therefore, the pH ceiling. Most salt, liquid chlorine and cal hypo pools should have 60-70 ppm TA.
- Adjust pH weekly into the yellow on the LSI. Never lower the pH enough to force the LSI to go red. If that occurs, etching of plaster (or damage to fiberglass and vinyl) can occur. Plaster etching will lead to a spike in pH due to the loss of calcium hydroxide.
If done correctly, if and when the pH naturally rises up to its pH ceiling, the LSI should still be balanced (in the green on the Orenda Calculator™).
Measure and dose acid correctly
For this pH containment strategy to work, the importance measuring and dosing acid correctly cannot be overstated. Lower alkalinity reduces the buffering capacity in the water, so measuring acid is imperative to avoid overcorrections, and it also matters that you pour acid correctly with dilution.
Related: Six Bad Habits that are Costing you Time and Money
Because each dose of acid will lower alkalinity––albeit slightly, when using this strategy correctly––every third or fourth week you will need to re-up the alkalinity using sodium bicarbonate. Contrary to conventional wisdom, 80-120 ppm total alkalinity is not always a good idea in a swimming pool.
Conclusion
Because of carbonate and bicarbonate alkalinity, the amount of dissolved carbon dioxide determines the pH of the water. The more dissolved CO2, the lower the pH, and vice versa. A pool's pH will naturally rise over time, thanks to CO2 off-gassing from the water. This is natural.
Henry's Law of solubility of gases explains why CO2 leaves until it is in equilibrium with the atmosphere. When that equilibrium is reached, the water flat, like a beer or soda that has had time for its CO2 equalize with the room. We call this moment of equilibrium the pH ceiling.
So the pH doesn't rise indefinitely, it just rises until the CO2 has equalized, and the pool goes flat. This is just one of many misunderstandings about pH.
We recommend being proactive and adopting a pH containment strategy, rather than chasing it and trying to control it. Use calcium hardness and Henry's Law to your advantage, and you will be able to predict pH rise accurately and consistently.
Note: We apologize for the inconsistent citation formats below, as some of these sources were difficult to find authors and publication dates. Use the hyperlinks to find the original sources.
1 Utah State University research paper (2006). Carbon Dioxide and Carbonic Acid.
2 It should be noted that the pH rise from non-stabilized chlorine like sodium hypochlorite (liquid chlorine) or calcium hypochlorite (cal hypo) is temporary, because when HOCl oxidizes or sanitizes, a byproduct of HCl offsets the high pH. Source: Robert Lowry.
3 Chemistry Libretexts. Henry's Law.
4 Carbonate alkalinity is confusing, because the term means two things. Carbonate alkalinity by itself is CO3, but for LSI and pH ceiling purposes, "carbonate alkalinity" refers to "corrected alkalinity", which includes bicarbonate, since you could simply remove a Hydrogen and still have a carbonate. Corrected alkalinity is Total Alkalinity minus cyanurate alkalinity, and if used, borates must be factored in too, because they also contribute to total alkalinity.
5 Wojtowicz, J. (1998). Swimming Pool Water Buffer Chemistry. Journal of the Swimming Pool and Spa Industry. Vol. 3(2), pp. 34-41.
6 Wojtowicz, J. (1996). Factors affecting the Calcium Carbonate Saturation Index. Journal of the Swimming Pool and Spa Industry. Vol. 1(2), pp. 9-15.
7 While the pH does not impact the chlorine strength (%HOCl) much in a stabilized outdoor pool, it DOES impact how well chlorine can stay bound to CYA and remain protected from sunlight degradation. As the pH rises, OCl- breaks away from CYA and can be destroyed from the sun. We talk about this in episode 128 of the Rule Your Pool Podcast:
