Calcium: The Most Misunderstood Chemistry in the Pool Business

Calcium hardness has a bad reputation, and that reputation is undeserved. When people see calcium scale or plaster problems that are white in color, they immediately assume it's calcium's fault. In reality, calcium is actually your best friend for water balance, as it is remarkably stable, and able to help you keep your pool in LSI balance year-round.
Covered in this article:
- Calcium hardness is underappreciated
- Calcium is the bedrock of the LSI
- LSI first, range chemistry second
- Conclusion
Calcium Hardness is underappreciated
Pick your pool surface: plaster, pebble, tile grout, fiberglass, or vinyl. What do they all have in common? They are all–without exception–impacted by the calcium saturation of the water. I'm writing this article to attempt to explain the urgent need to maintain sufficient levels of calcium hardness in our swimming pools.
Having begun my career in the pool service industry in 1984, I have a confession to make. Call it an admission of guilt. A common attitude–which I shared–was “I do pool repair, not chemicals”. Be honest with yourself: how many of us pool repair technicians even bother to carry a water chemistry test kit in our truck? For decades I didn't. How about bags of calcium chloride? No? Me neither.
Okay, let's be fair, maybe you have some test strips or a 4-in-1 test kit to check the pH and chlorine. But I have news for you, my fellow pool service technicians. When we discover a corroding heat exchanger and a copper-stained spa, the conclusion of "low pH" is not the entire story. And when we see calcium carbonate scale on the tile line or calcium flakes in saltwater pools, those are not caused simply by "too much calcium hardness." There are more factors at play.
I used to think that too, and I was painfully mistaken...at the expense of my customers and my own livelihood. You see, both etching and scale are actually determined by the Langelier Saturation Index (LSI) of the water. And calcium hardness is just one-sixth of the LSI equation. And mathematically, it's not even in the top three most significant factors of the LSI.
Calcium is the bedrock of the LSI
So what is the LSI? It's the international standard method for telling us how "balanced" water is, based on how saturated the water is with calcium carbonate (CaCO3). I know that's a mouthful, but you can listen to our Rule Your Pool Podcast for a more detailed explanation:
In short, the amount of calcium carbonate dissolved in water is measured as calcium hardness. But how saturated the water is with that calcium carbonate is the LSI. And it's the LSI that matters to water.
There are six (6) factors of the LSI: water temperature, pH, alkalinity, calcium hardness, TDS and CYA. Of those six factors, five of them determine how saturated the water is with the remaining factor, calcium hardness.
Water, like everything else in nature, craves equilibrium. Either we need to give water the balance it craves, or it will stop at nothing to balance itself. We have six factors to adjust, which gives us over 720 combinations of numbers to play with. Water has only two options to balance itself: eat or scale.
Water is water, regardless of what type of pool it's in
So you say to yourself “But Harold, I treat vinyl liner/fiberglass pools…the surface is not at risk.” Wrong. All surfaces are impacted by aggressive water, with one exception. Neither vinyl nor fiberglass has available calcium to donate to the water like a plaster pool. That is to say, in calcium-deficient water, a plaster surface gives up the necessary calcium to water so it can rebalance itself. Vinyl and fiberglass pools do not...but they do degrade.
Equally important to note: surface degradation is gradual and will escape visual evidence for quite some time…perhaps leading you to believe there is no damage taking place.
Low LSI water in vinyl or fiberglass pools stays corrosive longer. Therefore, fiberglass gel coats deteriorate and vinyl liners fade. Truth be known, vinyl liner and fiberglass pools are likely to decline faster than plaster in low-LSI water. And yet, manufacturers of such pool surfaces say the opposite.
This is based on the old, flawed paradigm of range chemistry being all that water cares about. The LSI is universal and does not care about our opinions. It is the objective, unbiased measurement of water balance.
Are you testing for calcium?
So if you're like many pool service techs, you might test for pH and chlorine on a pool visit. Well congratulations, you know the pH (for the moment, because pH naturally rises), and pH is just one-sixth of the equation. In other words, you have no idea if the water is actually balanced or not. So as professionals, we need to focus on what water wants, not what we want. We need to prioritize the holistic balance of water rather than individual chemistry ranges.
LSI first, range chemistry second
The pool industry has been force-fed range chemistry requirements for decades. The problem is not with the individual ranges themselves–though some of them seem arbitrary at best–it's that focusing on a few individual trees in the forest prevents us from seeing the forest itself. We need to instead focus on how all these factors tie together. And that's accomplished using the LSI. We build the free Orenda Calculator™ for this exact purpose.
My message to you is a simple one: focus on the LSI first, and then you can think about individual ranges second. But you will likely find that LSI balance is physically impossible at certain times of the year, especially in winter. Cold water lowers the LSI, and that means cold water is hungry for more calcium hardness. This means you will need to violate certain individual factors in order to preserve LSI balance throughout the year.
Water temperature matters!
When I first played around with our calculator app, I thought we had an error in our LSI formula. I simply could not believe what lowering the temperature did to the water. I had been screwing up pools for decades and had no idea. In fact, I was convinced that I had been doing it right all along...until the LSI calculator fed me a full serving of reality on a silver platter.
Check this out:
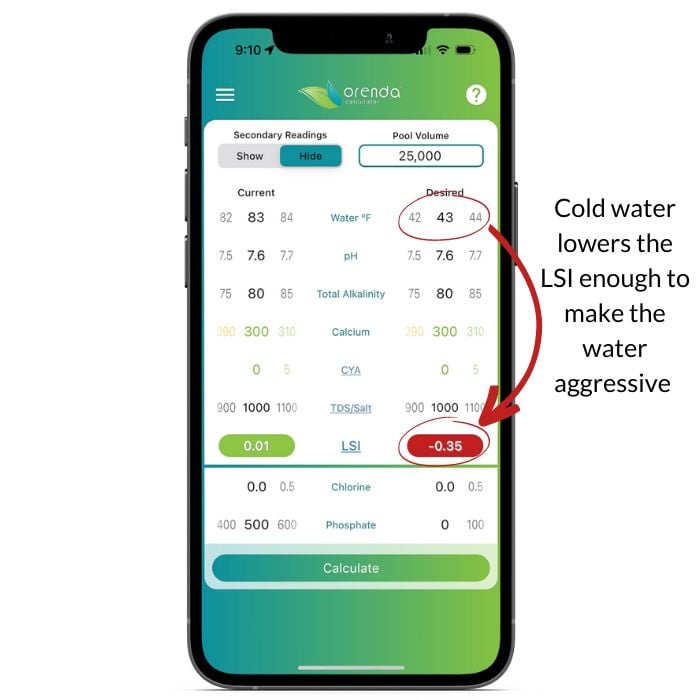
In my opinion, we choice is clear. We need to do the right thing for our customers and focus on the LSI. In the example above we have "textbook" perfect range chemistry. Just look at it! 7.6 pH, 80 ppm alkalinity, 300 ppm calcium. Textbook perfection. And yet, the temperature drops, and our water attacks the pool. Regardless if it's a cement finish, vinyl liner or fiberglass pool. Aggressive water damages all of them.
So what can we do? Lean on calcium hardness, the bedrock of the LSI:
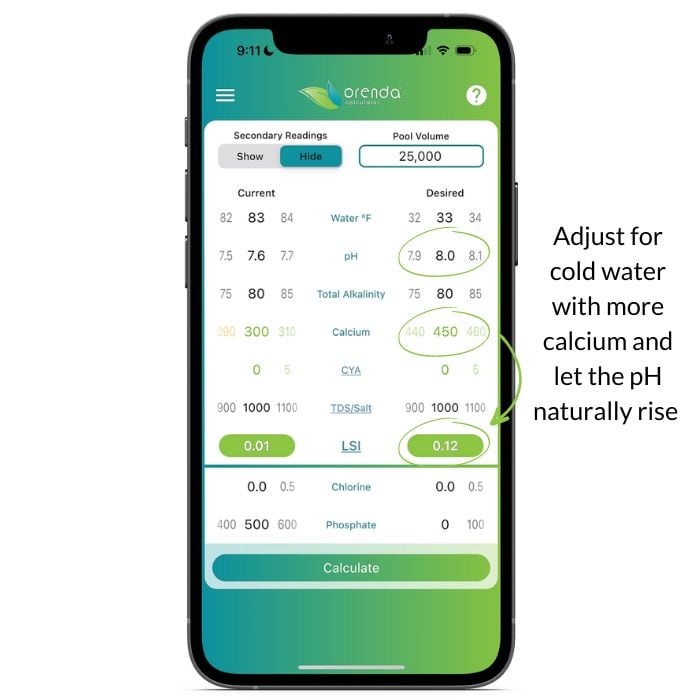 There are certain physics at play here that we explain more in-depth in other articles. In short, let your pH naturally rise (because it will unless you have a solid cover over the pool), and feed the pool calcium before it hibernates for the winter.
There are certain physics at play here that we explain more in-depth in other articles. In short, let your pH naturally rise (because it will unless you have a solid cover over the pool), and feed the pool calcium before it hibernates for the winter.
And you might be thinking, "high calcium causes scale!" Rarely. It's a high LSI that causes scale. And if you're thinking about those calcium crystals you might see when you re-open pools in the spring after a cold winter, those crystals are not scale.

High calcium is rarely a problem. It's usually a benefit.
For some reason, calcium is treated like a bad word in our industry, and I know why: we associate calcium hardness with scale. But that's just our own ignorance, and I'm personally as guilty as anyone. We don't know what we don't know.
The truth is, having more calcium hardness is a benefit in most pools. Sure, at a certain point there can be too much calcium in the water, which makes it difficult to maintain LSI balance. But up until that point, calcium is our best friend in water chemistry. It's reliable, stable, and never lets us down in when the temperatures drop. It is OUR responsibility to keep the water happy with the amount of calcium carbonate in it. That's not calcium's job. It's ours.
Conclusion
Calcium is a friend, not a foe. We need it, especially when water temperatures drop. It's the most misunderstood chemistry because it gets blamed for problems like scale. And frankly, many calcium issues in pools are mistakenly diagnosed as scale...and they are not! Issues like crystals, calcium nodules, efflorescence, winter dust and uneven carbonation are the most common calcium problems we hear about at Orenda, and none of them are actually scale. We touch on each of these issues in our article on understanding calcium hardness.
My point is this: test for calcium and make sure you have enough of it in your water. Water balance depends on it.

Harold Evans began his pool service/repair company, The Pool Surgeon, in 1984. Frustrated with problems he could continue to fix--but not prevent--he searched for a water chemistry solution to resolve many of the problems he faced as a pool professional. Harold is now the CEO/owner of Orenda Technologies and focuses on educating pool professionals on how to make the swimming pool safer, long-lasting and enjoyable for all.
