How to Use an Acid Feeder on a Swimming Pool

What is the recommended way to add acid to a commercial swimming pool? We have discussed how to add acid to a residential pool, but this article will focus on the consequences of incorrectly adding acid on a pool with chemical automation. Mainly, pool surfaces get etched–especially the floors. Spoiler alert: acid dilution is a smart solution.
Covered in this article:
- Chemical automation and pH "control"
- pH naturally rises
- Acid is denser than water
- Things to consider when using an acid feeder
- Dilute the acid
- How to dilute acid safely
- Install a CO2 injector system
- Consider using sodium bisulfate
- Dilute the acid
- Conclusion
We did not realize it until recently, but there is a direct correlation between how acid is fed into a pool, and the difficulty a pool has in maintaining alkalinity levels. Acid problems are common everywhere, and much of it is because muriatic acid is 1.18x the density of water (18% denser). 18 percent may not sound like a lot, but in water, it is a big enough difference to cause acid to plummet to the bottom of a swimming pool. Here's a photo of a muriatic acid dye test:

Credit: onBalance
Acid feeders are simple enough, but it's what we do before the acid feeds that matters. Acid needs to be fed differently than liquid chlorine, enzymes, and other chemicals like metal and scale inhibitors.
Chemical automation and pH "control"
A pool chemical controller has a pH probe that gives it a real-time reading of the water's pH. The controller is set to maintain a target pH within a certain range. Let's say in this example, the controller is set to 7.5 pH. When the pH climbs out of the specified range–say, 7.4 to 7.6–the controller sends a signal to the acid feeder to introduce liquid acid into circulation.
Warning: Acid and its fumes are highly corrosive and dangerous. Always use protective gear and use with caution. Acid should always be stored in a separate room from the rest of the pool equipment. If it is a residential pool, follow manufacturer guidelines for storing acid at a safe distance from other chemicals.
For indoor pools without cyanuric acid, pH commands the strength of chlorine, as defined by the percentage of strong Hypochlorous Acid (HOCl) relative to its weaker counterpart, the Hypochlorite Ion (OCl-). This relationship between pH and chlorine strength is very different in a stabilized pool, but for now, let's assume we're talking about an indoor commercial pool without stabilizer.
PDF Download: Relative Effects of pH and CYA on Disinfection - Stanley Pickens, Ph.D.
Muriatic acid will be fed through a feed pump multiple times a day to keep up with the pH swings. This includes temporary pH increases too, like when the chlorinator feeds add liquid chlorine or cal hypo.
As a result of this near-constant drip of acid going into the pool, commercial operators often struggle to keep alkalinity within range. They experience low alkalinity. They also experience rough pool surfaces, especially on the floor of the pool. What's really going on?
pH naturally rises
You may notice that pH "control" involves adding substances that only lower pH. There is no soda ash or sodium bicarb feeder. Why? Because pH naturally rises due to the loss of carbon dioxide. Just keep that in mind as you read further.
Acid is denser than water

In the gorgeous diagram above (which we made ourselves...no big deal), the blue/purple clouds represent influent water coming out of a floor return inlet. Commercial pools often have floor returns just like this throughout the pool, especially on skimmer pools. Since muriatic acid is 18% denser than water, it will stay low in the pool, creating a localized low-LSI violation, which will etch the surface (water steals calcium out of the cement). This localized problem leads to calcium carbonate being dissolved, and high pH calcium hydroxide being drawn out of the plaster surface and it neutralizes some of the acid...and as a result, raises the pH in the pool.
Related: Total Alkalinity vs. pH and their roles in water chemistry
The vicious cycle continues from there. Acid will continue to feed because the pH probe reads a rising pH, so on and so forth. This problem compounds itself when there are metals (like iron) in your water. Primarily, iron staining will most often present itself around return inlets.
 So how can operators get the pH control necessary, while minimizing its negative consequences? So far, we know of three strategies that can help.
So how can operators get the pH control necessary, while minimizing its negative consequences? So far, we know of three strategies that can help.
Things to consider when using an acid feeder on a swimming pool
1. Dilute the acid
We spoke to several commercial pool service professionals while doing our research for this topic. While they did not agree on everything, everybody agreed that diluting muriatic acid is a good idea. Depending on the feed pump and how it is programmed, acid is often added too fast. Sure, in a commercial pool, the return line can have 700+ gallons per minute (GPM) of flow. One would think that is plenty of dilution–and it does help–but it is not always enough. Dilution is a beneficial solution.
How to dilute acid safely
Acid dilution by hand is not advised. It is too dangerous, especially if the pool is large enough that it uses 15-gallon drums of acid (or larger). The safest way we know of to dilute acid is to feed the acid into a larger chemical holding tank that has water in it, and dilute to at least a 5:1 water to acid ratio. Just like normal acid storage, this dilution tank should be in a separate room from any other chemicals or equipment. Replenish the water with a hose or install a fill line to pour water into the tank in a controlled manner. The basic sequence of using a dilution tank is shown in our not-quite-as-gorgeous-as-the-previous-illustration representative diagram below.
 Caution: Do not install systems without a licensed professional. Acid is dangerous.
Caution: Do not install systems without a licensed professional. Acid is dangerous.
Obviously this diagram is only outlining the basic concept of how to set up a dilution tank. You can purchase a chemical holding tank from plastic manufacturers like this. Consult a licensed pool builder or service professional before deciding to to this. Your chemical controller will need to be modified for the diluted strength of your acid.
2. Install a carbon dioxide (CO2) system
Back to the experts we talked to about this topic. They each told us about their own problematic experiences with feeding muriatic acid on commercial pools. So they have mostly abandoned muriatic acid in favor of CO2 systems and Sodium Bisulfate (see item #3 below). They have noticed a few advantages.
 First, CO2 systems lower pH but do not lower alkalinity. Commercial pool operators that feed undiluted muriatic acid tell us they have problems maintaining alkalinity levels. CO2 will not reduce alkalinity...if anything, pool operators may notice alkalinity rises using CO2. At this time we have not done enough research to explain that correlation, but all of the people we spoke to attested to a rise in alkalinity when injecting CO2 into their pools. We presume it has something to do with additional carbonic acid (H2CO3), but we do not know yet.
First, CO2 systems lower pH but do not lower alkalinity. Commercial pool operators that feed undiluted muriatic acid tell us they have problems maintaining alkalinity levels. CO2 will not reduce alkalinity...if anything, pool operators may notice alkalinity rises using CO2. At this time we have not done enough research to explain that correlation, but all of the people we spoke to attested to a rise in alkalinity when injecting CO2 into their pools. We presume it has something to do with additional carbonic acid (H2CO3), but we do not know yet.
Related: What is Alkalinity?
Additionally, from a safety perspective, if a feeder goes haywire and feeds CO2 without stopping, the lowest pH will be in the 6 to 7 range. Acid, however, can reduce the pH to dangerously low levels if there is a malfunction. And CO2 needs to be stored and handled properly, because if it leaks in a confined area (like the pump room), it can displace oxygen and could be a hazard. So be aware of it, and get a CO2 detector in the space.
All that said, we are recommending exclusively using CO2 systems. These pools being discussed still have an acid feed system to lower alkalinity when necessary. You can program your chemical controller to decide which to feed (acid or CO2) based on the alkalinity at the time. For example, if the total alkalinity is over 100 ppm, the controller logic would trigger the acid feeder. If the alkalinity is below 100, it would feed CO2 instead. All these types of parameters are decided by the technician programming the system. Maybe you want your alkalinity threshold 80 ppm instead of 100. The tap water chemistry should be considered when making such decisions.
3. Consider using Sodium Bisulfate
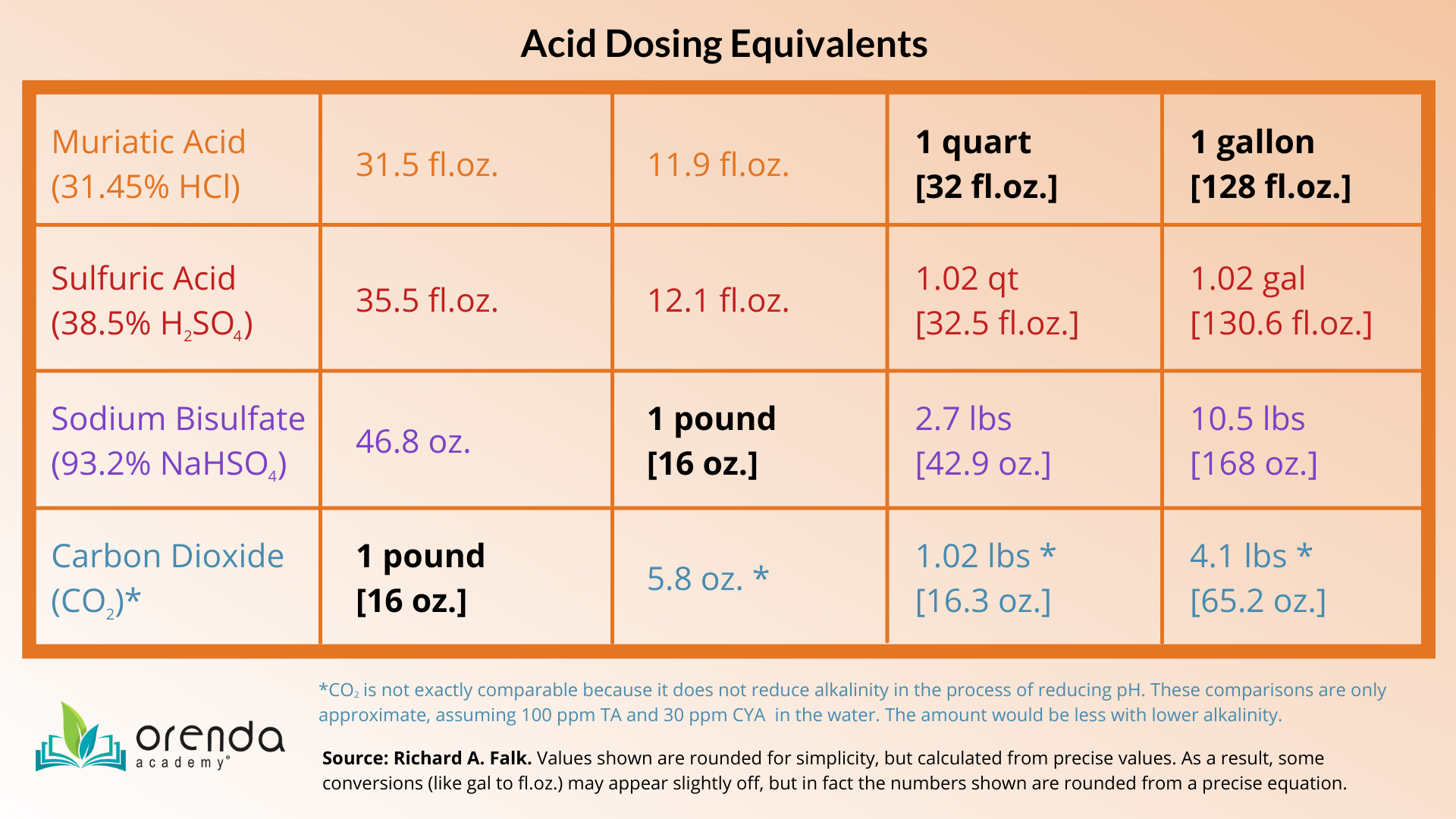

Second, commercial pools struggling to maintain alkalinity can consider switching from muriatic acid to sodium bisulfate (NaHSO4). While sodium bisulfate is denser than muriatic acid, it is a dry acid that must be pre-dissolved and diluted. This pre-dilution lowers its density and allows for it to be fed into circulation using a feed pump. Sodium bisulfate's pH is not as low as muriatic acid's, so it is a less aggressive acid that still accomplishes pH and alkalinity reduction. While sodium bisulfate on a feeder may not be ideal for residential pools, it may be a good alternative for commercial pools with chemical automation.
Sodium bisulfate is not without its negatives too, like the byproduct of sulfates getting into the water, and the eventual risk of calcium sulfate scale. Sulfates alone are enough reason to schedule regular dilutions of your water to keep sulfate levels below 300 ppm. Also, be aware that pre-dissolved sodium bisulfate takes more work unless you have an automated feeder. Costs vary depending on your location and supplier.
 Beware of calcium sulfate scale, especially if using sodium bisulfate on a pool with cal hypo chlorine. Calcium sulfate scale is much more difficult to remedy than calcium carbonate, as muriatic acid does not dissolve it in normal conditions.
Beware of calcium sulfate scale, especially if using sodium bisulfate on a pool with cal hypo chlorine. Calcium sulfate scale is much more difficult to remedy than calcium carbonate, as muriatic acid does not dissolve it in normal conditions.
Conclusion
In residential pools, abusing acid is the #1 bad habit we see in the entire pool industry. In commercial pools, the same principles still apply, though acid is generally added through an automated or timed feed pump. If undiluted muriatic acid is fed directly into circulation, pools can struggle to maintain alkalinity levels, and often experience etching of their plaster surface–especially around the floor return inlets. This can actually lead to a spike in pH near the surface because the acid stays low in the pool and creates a localized LSI violation, which draws high-pH calcium out of the cement. It's basically an automated overcorrection of pH that will cost pools in more chemical consumption and frustration.
To avoid these issues, we recommend diluting acid prior to feeding it. This can be done with a larger holding tank, much like a liquid chlorine storage tank. However you decide to dilute the acid, do so with caution! Acid is very dangerous, as you know.
Another good alternative is to install a CO2 system for pH reduction, which will not lower the alkalinity. You still need acid when you have to lower alkalinity, but CO2 will probably be used most of the time.
Finally, if the issues continue, consider switching from muriatic to sodium bisulfate acid, BUT with regular dilution of your water to handle the sulfates. Sodium bisulfate is a dry acid that must be pre-dissolved and diluted, but the commercial pool experts we spoke to are making the switch for their own valid reasons. They know far more than we do, and according to them, the benefits far outweigh the costs.
