Types of Calcium Dust in Pools

There are three distinct types of calcium carbonate (CaCO3) commonly found in swimming pools: calcium carbonate scale, calcium (calcite) crystals, and various forms of calcium dust. This article focuses on calcium dust.
Covered in this article:
- What causes white dust in a pool?
- Plaster Dust
- Winter Dust
- How to prevent winter dust
- Carbonate Clouding
- How to prevent carbonate clouding
- Conclusion
What causes white dust in a pool?
White dust in a swimming pool can happen for several reasons. Not all white powders are the same. It could be:
- Diatomaceous earth (if you have a DE filter with a broken grid)
- PR-10,000 precipitates a cloud of inert white dust as part of the phosphate removal process
- Various undissolved dry chemicals (like cal hypo, calcium chloride, trichlor or dichlor)
- A fallout of calcium carbonate due to a high-LSI violation
- Soda ash can force an LSI violation and cloud the water
- Sodium bicarbonate can also force an LSI violation and cloud the water
- Increasing temperature in the pool or spa rapidly can cause cloudy water that precipitates calcium carbonate dust to the floor
- Saltwater pools can have calcium carbonate flakes that can eventually disintegrate into white dust if the water is circulating enough
- Etching of cement can force a localized LSI violation
- Low-LSI water etches and pulls out calcium hydroxide from the cement, which spikes the pH near the surface, forcing a high-LSI condition temporarily, which carbonates the calcium hydroxide into calcium carbonate dust.
- This is known most often as plaster dust, but winter dust is formed in the same way
- Low-LSI water etches and pulls out calcium hydroxide from the cement, which spikes the pH near the surface, forcing a high-LSI condition temporarily, which carbonates the calcium hydroxide into calcium carbonate dust.
White dust is usually some type of calcium carbonate (CaCO3). The calcium carbonate either came from an oversaturation in the water or was drawn out of the cement in a pool finish. If you have a vinyl liner or fiberglass pool, the dust is likely from an oversaturation of calcium carbonate (+0.31 LSI or higher).
Just because a pool surface or a substance on it is white does NOT mean it is calcium carbonate. For instance, most plaster discolorations are not scale. They are caused by etching, which pulls calcium hydroxide to the surface, which then carbonates pure white.
Fiberglass pools can have a condition we call chalking, which is NOT scale. Our theory is that chalking is the deterioration and oxidation of the gel coat.
Now, let's examine calcium dust more closely and distinguish between four types: plaster dust, startup snowing, winter dust, and carbonate clouding.
Plaster Dust
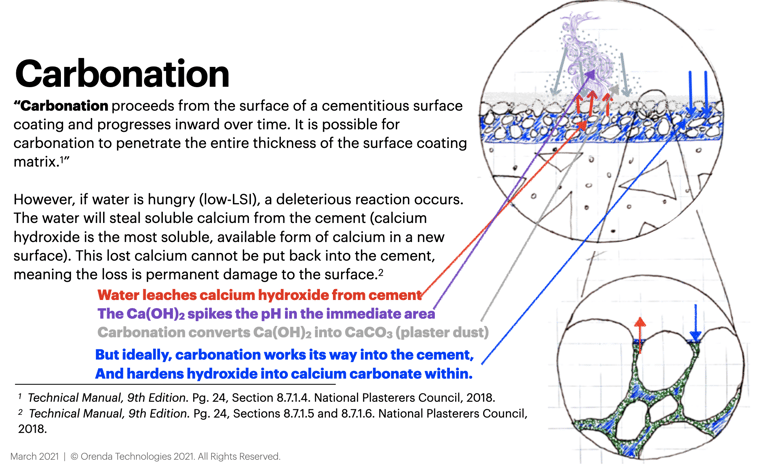
Plaster dust occurs when low-LSI water fills a newly plastered pool and begins dissolving calcium from the cement.
Within the cement, the most soluble form of calcium is calcium hydroxide, Ca(OH)2. Calcium hydroxide has a high pH of about 12.6. When brought into solution, it rapidly changes the pH of the water near the surface, dramatically raising the LSI in that immediate area. This increase in LSI pushes calcium carbonate out of solution in the form of dust.
The 12.6 pH of calcium hydroxide can force calcium carbonate out of solution in two ways:
- The high pH and high calcium concentration create a high LSI violation, which is a scale-forming condition.
- The high pH is well above 8.3, which means bicarbonate ions (HCO3-) in the water convert into carbonate ions (CO32-). Carbonate ions are attracted to calcium ions to form calcium carbonate (CaCO3).
Ca2+ + CO32- → CaCO3↓calcium ion + carbonate ion → calcium carbonate (↓ precipitated)
Plaster dust is hard evidence that valuable calcium was stolen from the cement in the surface; cement that has not yet had time to fully hydrate and cure.
Anyone who has done several plaster startups has probably noticed how calcium tends to “drift up” during startup, and the pH spikes high for the first several days you come back to the pool. These things happen because the pool water is stealing high-pH calcium hydroxide from the new plaster finish.
 Plaster dust being brushed. No matter what the cement pigment color is, plaster dust is pure calcium carbonate, and therefore it is always pure white.
Plaster dust being brushed. No matter what the cement pigment color is, plaster dust is pure calcium carbonate, and therefore it is always pure white.
How to prevent plaster dust
Preventing plaster dust is simple: prevent the water from stealing calcium hydroxide from cement.
That's it.
How can that be accomplished when the chemistry of the fill water is different everywhere? The first part is actually testing the tap water (or truck water) that fills the pool when it gets resurfaced. If we don't know the chemistry and LSI of the fill water, we have no idea what the water is missing to satisfy its need for equilibrium.
The Orenda Startup™ method is an LSI-based startup that focuses on giving the water the LSI balance it craves so it will not have the ability to steal calcium from the cement.
Related: The Orenda Startup™ Step-by-step Procedure
If we don't balance the water, it will have to balance itself––at the expense of the calcium in the cementitious pool finish (like plaster, pebble, quartz, etc.).
Startup snowing
During a startup, if the water is undersaturated with calcium carbonate (meaning the LSI is below -0.30), it will find calcium wherever it can get it easiest. But what if you're doing the Orenda Startup™ or the onBalance Bicarb Startup™ and you get white dust on the pool floor?
It could be what we call snowing, or startup snow.
Snowing is the consequence of creating a high-LSI violation (by mistake) when filling the pool. This can happen either by adding too much calcium (or sodium bicarbonate), adding it too fast, or adding it to water with too high of a pH. We have seen it happen for each of these reasons, and sometimes a combination of them.
If calcium dust is on the floor during a pool startup, there are three ways to identify whether it is plaster dust or startup snow.
- Test the pH. Did it spike well above the pH Ceiling? If so, it's likely plaster dust because a very high pH accompanies plaster dust. This is because it was created by water stealing 12.6 pH calcium hydroxide from the cement. If there is no spike in pH, it's not plaster dust.
- Look at the walls. If it's plaster dust, there should be evidence of some dust still stuck to the vertical walls and edges of the pool, not just the floor. If it is startup snow, it will only be on the floor because it came from the water and fell to the floor. Plaster dust comes out from within the plaster itself.
- Test the calcium hardness. If it is plaster dust, the calcium level should be at or even above where you were aiming when you filled the pool. If it is startup snow, you are looking at much of the calcium you added during the fill-up, so the calcium level will be noticeably lower than where you were aiming it to be.
Winter Dust

Let’s say you winterize a pool and come back in the spring to open it. Have you ever seen dust in your pool, or perhaps what you think is “scale” at the bottom of the pool? This calcium may be stuck on your plastic fittings, too. This is similar to plaster dust, except the plaster was already cured. This sort of dust can occur with surfaces many years old and seems to only occur in very cold water (40ºF or colder in the winter).
You will see small white rings around aggregate in with this condition, which indicates the loss of--you guessed it--calcium hydroxide. The rings occur because this calcium hydroxide is most often found in the interfacial transition zones (ITZs) between aggregate. Rings around aggregate mean the calcium in the ITZ's has been stolen and replaced with more calcium hydroxide, which has then carbonated again into white calcium carbonate.

This high-pH calcium hydroxide spikes the pool's pH, and eventually, the water stops eating the walls because the pH provides temporary LSI equilibrium through the winter. However, unlike plaster dust, the precipitation of calcium carbonate does not happen immediately. Rather, the dusting is delayed, and we think this happens because of warming water temperature.
We think the precipitation occurs as the water goes from freezing to, say, 60ºF by the time you get there in the spring. The water already stole the calcium hydroxide and spiked the pH, and high pH in warmer water begins to precipitate calcium carbonate as dust.
For some reason, this form of calcium dust is often mistaken for carbonate scale. This dust formed for a different reason, but technically, as the water warmed up, the LSI changed and precipitated calcium carbonate out of solution. So sure, if you want to call it scale, you can, but we advise against it.
How to prevent Winter Dust
Bad habits have developed in cold climates, like trying to keep calcium low going into the winter to prevent scale. Rule of thumb: if you see it on the bottom of your pool, it wasn't a high-LSI problem like scale. It was a low-LSI problem. Preventing winter dust is as simple as giving the water enough calcium and alkalinity so that it's LSI will remain balanced in the coldest temperature during the winter.
If the pool will freeze, balance the LSI for 32ºF (0ºC). This should prevent the water from being able to steal calcium from the cement in the first place.
Carbonate Clouding
 A third type of calcium dust is created when adding chemicals improperly. One way is when you add soda ash, either too fast or too much.
A third type of calcium dust is created when adding chemicals improperly. One way is when you add soda ash, either too fast or too much.
This drives up the pH in its immediate area, converting bicarbonate ions to carbonate ions. These ions then attract calcium and create calcium carbonate. Calcium ions are strongly attracted to carbonate ions (as mentioned earlier).
How to prevent carbonate clouding
To avoid this, we recommend pre-dissolving chemicals before adding them to a pool and not adding sodium bicarbonate, soda ash, calcium chloride, or calcium hypochlorite to water that has a pH above 8.0. Before adding such chemicals, use diluted muriatic acid to get the pH below 8. We like to see it below 7.8 for best results.
Apart from that, general LSI balance in the pool should be maintained, which is the action step of our first pillar of proactive pool care.
Conclusion
This article describes a few distinct forms of calcium dust: plaster dust, winter dust, startup snowing, and carbonate clouding. These forms of dust happen for different reasons, and prevention is easier than remediation.
Sure, acid will dissolve all of these forms of calcium carbonate, but it matters how the dust got there in the first place. Once you know that, you can use the free Orenda Calculator™ to figure out the proper correction strategy before the problem starts. Do your part with the LSI in preparation for changing temperature or other conditions, and SC-1000 can help you do the rest. If you need help, we would love to hear from you.


