Sulfuric Acid, Sodium Bisulfate & CO2 | Muriatic Acid Alternatives

The 2021 chlorine shortage has brought about an acid shortage too. As a result, pool owners, builders, plasterers, and service professionals may need to find alternatives to muriatic acid until the supply chain gets caught up. This article will introduce you to CO2 and two acid alternatives.
Covered in this article:
- A quick reference dosing table
- Why acid is needed in pools
- Carbon dioxide (CO2)
- Sulfuric acid
- The difference between muriatic acid and sulfuric acid
- Sodium bisulfate
- Sodium bisulfate vs. muriatic acid
- Sulfates
- How to reduce sulfates in pools
- Pool startup chemicals
- Conclusion
Why is acid used in pools?
Acid is used in pools to lower pH and reduce total alkalinity as needed. Acid has a high concentration of Hydrogen ions (H+), and when added to water, it converts bicarbonate ions (HCO3-) and carbonate ions (CO32-) into carbonic acid (H2CO3). Carbonic acid is dissolved CO2(aq), which lowers the pH. For a helpful visual, when acid is added, move left along the chart to see how the equilibrium of carbonates changes:
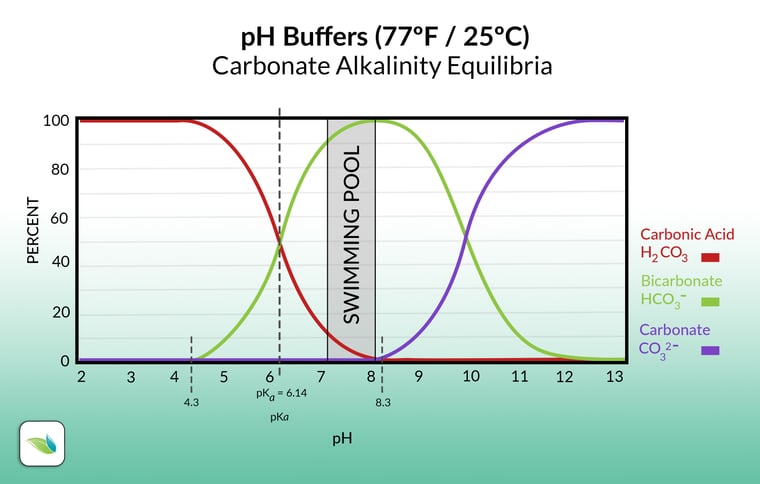
Without acid to reset the naturally rising pH, swimming pools would be operating at a pH above 8.0 in most cases. That makes it challenging to maintain a balanced LSI, especially in the summertime when the water is warm. And in non-stabilized pools, a high pH means a lower percentage of Hypochlorous acid (%HOCl), which means weaker chlorine.
Related: Total Alkalinity vs. pH and their roles in water chemistry
The most common acid in pools is muriatic acid. Muriatic acid is a diluted form of Hydrochloric Acid (HCl). The muriatic acid most commonly used in pools is 31.45% HCl. Regardless of the acid you use, measure and dose it correctly and dilute it prior to adding it to the pool. Here's a video showing why dilution is so essential:
It's 2021, and due to the chlorine shortage, there is now a muriatic acid shortage. So let's talk about some muriatic acid alternatives in the event you cannot get what you need.
Carbon dioxide (CO2)
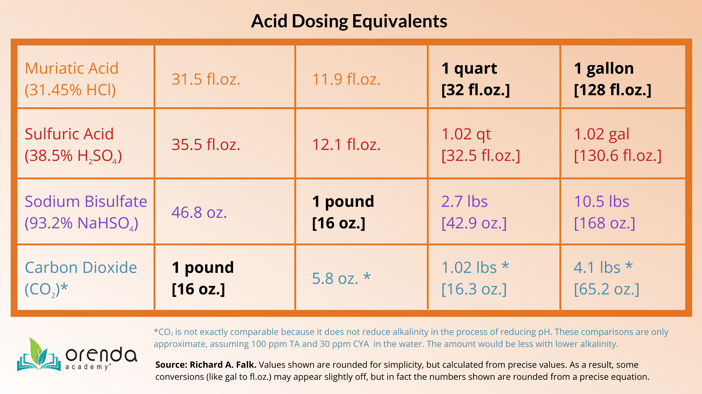
 Dosage comparison: 1 pound (16 oz.) of CO2 = 32.8 fl.oz. of 31.45% HCl.
Dosage comparison: 1 pound (16 oz.) of CO2 = 32.8 fl.oz. of 31.45% HCl.
It takes 3.9 lbs of CO2 to equal 1 gallon of muriatic acid's pH impact, but CO2 will not reduce alkalinity.
Carbon dioxide (CO2) is perhaps the purest, safest pH reducer on the planet. CO2 is injected using pressurized tanks and a feeder system. It is most common in commercial pools with chemical automation controllers. CO2 lowers pH by simply increasing the amount of aqueous CO2 in the water, called carbonic acid (H2O + CO2 → H2CO3). Unlike acid, CO2 will not lower alkalinity because it does not convert bicarbonate ions (HCO3-) into carbonic acid. In fact, alkalinity tends to rise in CO2-fed pools.
One pound of CO2 is roughly equivalent to one quart of 31.45% muriatic acid. And CO2 is usually a more affordable option for pH containment when compared to any acid, especially on larger pools. However, it should be noted that the costs of a CO2 system depend on how you get the CO2 to the pool. If you have to buy a CO2 tank and replace it each time, it can be costly. But if your tanks can be swapped or refilled, like on large commercial pools, CO2 is very economical. For more on CO2, read this.
Sulfuric Acid (H2SO4)
 Dosage comparison: 1.02 fl.oz. of 38.5% sulfuric acid = 1.00 fl.oz. of 31.45% HCl.
Dosage comparison: 1.02 fl.oz. of 38.5% sulfuric acid = 1.00 fl.oz. of 31.45% HCl.
It takes 2% more sulfuric acid to equal muriatic acid (by volume) to accomplish identical pH and alkalinity reduction.
Sulfuric acid is a comparable alternative to muriatic acid. And while these two acids have a very similar impact on pH and alkalinity, they are not the same. The main difference is the byproduct left behind. Muriatic acid leaves behind chlorides (Cl-), which are relatively innocuous since all chlorine types leave behind chlorides. Sulfuric acid leaves behind sulfates, which become problematic over time. We will go into depth on sulfates later in this article.
One gallon of sulfuric acid leaves behind 47.1 ppm sulfates in 10,000 gallons of water.
Differences between sulfuric acid and muriatic acid
Let's start with product concentrations and dosing. Because we are not chemists, we dug in and did some research.1 According to a chemistry forum, muriatic (31.45% HCl) is technically stronger, though it depends on the concentration you're buying. Fortunately, muriatic acid and standard 38.5% sulfuric acid are within 2% of each other, so dosing is virtually identical:
31.45% Muriatic acid dose x (1.02) = 38.5% Sulfuric acid dose
example:
32 fl.oz. of 31.45% Muriatic acid = 32.64 fl.oz. of 38.5% Sulfuric acid
Bonus: sulfuric acid and calcium chloride
And if you have ever tried to dissolve calcium chloride in a bucket of water with some sulfuric acid, you have probably noticed a strange phenomenon. Unlike when adding muriatic acid to the dissolving calcium, adding sulfuric acid seems to force undissolved calcium chloride to float to the surface. We do not know why, but here are some screenshots from a video of it:

Now, let's move onto sodium bisulfate.
Sodium Bisulfate (NaHSO4)
 Dosage comparison: 1 pound (16 oz.) of 93.2% sodium bisulfate = 12.1 fl.oz. of 31.45% HCl.
Dosage comparison: 1 pound (16 oz.) of 93.2% sodium bisulfate = 12.1 fl.oz. of 31.45% HCl.
Since sodium bisulfate is dry, weight and volume can be confusing. Follow dosing instructions on the product label.
Sodium bisulfate (Sodium Hydrogen sulfate) is commonly referred to as "dry acid" in the pool industry. A sodium bisulfate pool system generally involves a specialized feeder to dissolve the acid in the water, then feed the acidic solution into circulation. Commercial pools have brands like AcidRite® and AcidPlus®. But sodium bisulfate is actually more common on residential pools, as it is readily available in most retail pool stores. It is sold as a dry granular product in a bucket. To use it, measure the amount needed, then pre-dissolve in a bucket of water. Once dissolved, pour around the perimeter of the pool. Do not add undissolved sodium bisulfate to your pool, and especially do not let it sit on the pool surface. Always pre-dissolve it in a bucket of water.
While it's widely available on the market today, we will not be surprised if dry acid becomes scarce in the coming months until the supply chain issues are resolved. That's in part because the ingredients needed to create sodium bisulfate include sulfuric acid:
NaCl + H2SO4 → HCl + NaHSO4
Salt + Sulfuric Acid → Hydrochloric Acid + Sodium Bisulfate
Sodium bisulfate vs. Muriatic Acid
Sodium bisulfate is sold in the pool industry at 93.2% strength. And while that sounds high, it is still a weaker acid by volume than muriatic. According to onBalance and other sources, 10.5 pounds (168 oz) of sodium bisulfate is roughly equivalent to one gallon (128 fl.oz.) of muriatic acid. So for every ounce of muriatic acid, you need ~1.31 ounces of sodium bisulfate to do the same thing. This partly explains why sodium bisulfate costs more than muriatic acid. See the dosing chart above for reference.
Despite higher costs, sodium bisulfate is a safer acid to handle than both muriatic and sulfuric. But because it leaves behind sulfates and sodium, TDS rises somewhat rapidly when using sodium bisulfate.
One pound of sodium bisulfate leaves behind 9.6 ppm of sulfate in 10,000 gallons of water. For comparison with sulfuric acid on an equivalent dose:
In 10,000 gallons of water...
1.02 gallons of 38.5% sulfuric acid = 10.5 pounds of 93.2% sodium bisulfate
[(1.02) x 47.1 ppm] vs. [(10.5) x 9.6 ppm]
48 ppm sulfates vs. 100.8 ppm sulfates
So an equivalent dose of sodium bisulfate leaves behind 100.8 ppm sulfates, compared to 48 ppm sulfates left behind by sulfuric acid. That's more than double (2.1x) the sulfates for an equivalent dose of acid! We can convert in reverse to double-check our math. This time, instead of converting to one sulfuric gallon, let's convert to one pound of sodium bisulfate:
In 10,000 gallons of water...
1 pound of 93.2% sodium bisulfate = 12.1 fl.oz. of 38.5% sulfuric acid
[9.6 ppm sulfates] vs. [47.1 ppm ÷ (128 ÷ 12.1)]
9.6 ppm vs. [47.1 ppm ÷ 10.57]
9.6 ppm vs. 4.45 ppm
Whew. Okay, the math checks out. 9.6 ÷ 4.45 = 2.15. So yes, for an equivalent dose, sodium bisulfate leaves behind 2.15x the sulfates than sulfuric acid does. That explains why the UK's Pool Water Treatment Advisory Group (PWTAG) advises against sodium bisulfate as a primary acid, which will segway us into our next topic. From the PWTAG website:
"The total dissolved solids (TDS) need not be high for sulphate attack to occur if sodium bisulphate is used in pool water. Sulphate attack occurs under balanced water conditions, even at concentrations below the maximum recommended concentrations, but more slowly since the rate of attack is concentration dependent. Note also that sulphate ions can migrate into the cement mortars, renders, screeds or concrete and begin to react. However the effects will not be immediately evident as they manifest on the surface of mortars in contact with the pool water." [emphasis added]
Sulfates
So what are sulfates anyway? Honestly, before writing this article, we did not really know. We had heard of sulfates from the perspective of two problems in pools: corrosion and calcium sulfate scale. But there are more. According to the UK's water advisory group PWTAG, sulfates should not exceed 300 mg/L (ppm).2 Here's an excerpt from the PWTAG website on sulfate levels:
Clause 7.3.2.1 General
Ideally the sulphate concentration (SO42-) of water in swimming pools should not exceed 300 mg/L. Where greater concentrations of sulphates cannot be avoided, impermeable adhesives and grouting materials that are not affected by sulphates should be used. High levels of sulphate would otherwise react with and erode materials containing Portland cement (CEM 1). - PWTAG [emphasis added, and the original quote had SO3 instead of SO42-, which, according to Richard Falk, is a typo].
300 ppm (or mg/L) is a surprisingly low threshold considering how much sulfate is left behind by sulfuric acid and sodium bisulfate. If you're using these acids regularly, you should plan for regular dilution cycles.
Sulfates are rarely introduced to water naturally, and if so, it's usually in small amounts, like if the pool is filled with well water. Normally sulfates are introduced by various products. Obviously sulfuric acid and sodium bisulfate, but also the non-chlorine oxidizer shock potassium monopersulfate, and the algaecide copper sulfate.
Now let's explore sulfates through the lens of the problems they cause.
Sulfate concrete attack
A quick online search of 'concrete sulfate attack' shows a plethora of information about how sulfates destroy cementitious materials like pool plaster and concrete. It's common knowledge in the concrete industry. According to Richard Falk, at a molecular level, a sulfate ion (SO42-) is quite similar to a carbonate ion (CO32-). When sulfate levels are high enough, the sulfate ion overtakes carbonate as the dominant ion and can break down calcium carbonate (CaCO3) by figuratively knocking carbonate off of calcium (Ca2+) and replacing it. This new compound is called calcium sulfate, which we will explain in a moment. The bottom line: sulfates destroy concrete, even if the LSI is balanced. The higher the sulfates, the faster the rate of etching. See footnotes 2 and 3.
Sulfate and chloride corrosion of metals
Studies have shown a direct correlation between higher sulfate levels and corrosivity in water, even if the water is otherwise balanced on the LSI or Ryznar corrosion index. In particular, corrosion of metals accelerates when both chlorides (Cl-) and sulfates are present in water. According to the EPA, sulfates compound corrosion issues when chlorides are present. Here's an excerpt from their report:
"In some cases, sulfates seem to aggravate the effects of chlorides. Chlorides present in amounts as little as 0.3% with sulfates present can produce severe corrosion. Even quite low concentrations of chlorides can cause corrosion when concentrated by occlusion in surface films." - EPA.gov 4
More on sulfate-accelerated corrosion of common pool equipment here. Most pool owners seldom think about metal components in their water, whether it's ladders, gutters, or light fittings. But also do not forget about any metal components in circulation too, such as heat exchangers, copper pipes, and cast iron strainer baskets or valutes. All of these components are more likely to be corroded when sulfates are high.
Calcium sulfate scale
As mentioned a moment ago, when sulfates become the dominant ion in your water, sulfates can replace calcium carbonate with calcium sulfate. This destroys calcium carbonate in your pool surfaces and tile grout, but also can eventually lead to a severe scale issue. Similar to how the LSI predicts when water will form a carbonate scale (an over-saturation of calcium carbonate) when there's an over-saturation of calcium sulfate, a pool can begin to get calcium sulfate scale. This is a far more serious problem than the carbonate scale for two reasons. First and foremost, unlike the dissolving carbonate scale, the calcium sulfate scale cannot safely be chemically removed.5 In fact, thanks to onBalance, we have some great photos of what the calcium sulfate scale looks like: sharp crystals.

Calcium sulfate scale looks like crystals. This is a section of tile and plaster cut from a pool that had to be resurfaced. There is no easy chemical solution to remove the calcium sulfate scale. Photo courtesy of onBalance.5
Secondly, the crystals are sharp and sometimes almost invisible. They are notorious for cutting people's skin viciously. Here's another photo to show you what we mean:

How to reduce sulfates in pools
Drain and dilute. It's just like high CYA or high TDS. Dilution is the best solution.
Pool Startup Chemicals
Before wrapping this article up, we need to make one note about pool startups. Every plaster startup involves at least some acid. Yes, that includes the Orenda Startup™, which uses only enough acid to maintain LSI balance throughout the first 30 days. Traditional startups use a lot more acid than our process. So that begs the question: is sulfuric acid appropriate for startups? We think not.
We are officially saying no, do not use sulfuric or sodium bisulfate in the Orenda Startup™ process. Our process calls for industry-standard muriatic acid (31.45% HCl). We do not want to complicate the already-delicate water balance by introducing corrosive sulfates when the plaster is in its infancy.
That being said, after 30 days if your goal is to 'clean up' the surface by etching it uniformly, sulfuric acid could work...just be sure to think about dilution after the process to get those sulfates reduced. We advise against using sulfuric or sodium bisulfate to do a hot start, acid bath, or other low-pH treatment. The sulfates may cause more harm than good. Stick to muriatic acid.
Conclusion
If you are unable to get muriatic acid, the next best alternative for pH and alkalinity control depends on what is available to you. For pH reduction, CO2 is the most affordable and safest choice, except for the initial installation costs of the feeder system. Additionally, CO2 only reduces pH, not alkalinity. So that leaves us with sulfuric acid and sodium bisulfate. Feel free to use the quick reference dosing chart above whenever you need it (this article will live forever on this blog, and therefore in the Orenda App in the articles section of the main menu.
As long as you dose the acids correctly, measure them and dilute them prior to adding to the pool, you can accomplish pH and alkalinity reduction successfully. But be aware of the sulfates left behind! If you are using sulfuric or sodium bisulfate, it is very important to regularly dilute your water to keep your sulfate levels below 300 ppm. Corrosion of metals and deterioration of cement will still happen but at a slower rate. In extreme cases, high sulfates can lead to calcium sulfate scale, which usually requires physical grinding to remove, or chipping out the plaster and resurfacing the entire pool. It's a costly consequence of high sulfates in your water.
Good luck to you in this acid shortage, and we hope this article was valuable for you.
1 Our browser window currently has 26 open tabs. 25 of them are chemistry resources about this topic, and the 26th tab is this article. We hyperlink to many of them in this article. So if we get something wrong, let us know by contacting us and we'll correct it immediately. We're doing our best to simplify this and get it right.
2 Pool Water Treatment Advisory Group (PWTAG) is an authority on water chemistry in the United Kingdom. Here's what they published about sulfates: https://www.pwtag.org/sulphate-attack-february-2011/
3 Bensted, Rbrough and Page, et.al. Sulfate Attack.Topic on ScienceDirect.com, originally from Durability of Concrete and Cement Composites. 2007.
4 Report on the Corrosion of Certain Alloys. July 2001. EPA Office of Prevention, Pesticides and Toxic Substances.
5 Calcium sulfate scale: CaSO4 · 2 H2O. Here is a case study by Que Hales of onBalance trying to remove it from a pool in Arizona: https://aquamagazine.com/service/the-mystery-of-the-pointed-crystals.html
