How to Prevent Scale in Pool Heaters and Salt Chlorine Generators

Calcium carbonate scale forms in swimming pools and other water systems when the water is over-saturated with calcium carbonate. This can be measured and predicted using the Langelier Saturation Index (LSI). This article discusses why saltwater chlorine generators and pool heaters tend to scale more frequently than the rest of the pool. Hint: temperature matters.
Covered in this article:
- What causes scale?
- Importance of the LSI factors
- Scale in salt chlorine generators
- Salt-generated chlorine is pH neutral
- How to prevent scale in a salt chlorine generator
- Balance the water
- Chelate the calcium with SC-1000
- Cool down the salt cell
- Calcium phosphate scale
- Scale in pool heaters
- How to prevent scale in a pool heat exchanger
- Bonus: Other forms of scale in pools
- Calcium phosphate scale
- Calcium sulfate scale
- Conclusion
What causes scale?
Scale is when a calcium compound becomes oversaturated in water and begins to fall out of solution. It can harden on surfaces into what we call scale. The most common form in swimming pools and spas is calcium carbonate scale. The saturation of calcium carbonate is quantified and predicted using the Langelier Saturation Index (LSI), which we can easily calculate using the free Orenda Calculator™ on the Orenda App.
Other forms of scale will be discussed in this article, including calcium phosphate and calcium sulfate scale. In all of these cases, the calcium ion (Ca2+) is bound to an anion like carbonate (CO32-), phosphate (PO43-), or sulfate (SO42-). These are called ion pairs. As mentioned before, calcium carbonate (CaCO3) is by far the most common in swimming pools and spas. So let's focus on it for now.
The LSI tells us how saturated the water is with calcium carbonate. An LSI value of +0.31 and above means the water is oversaturated and likely to deposit scale on pool surfaces and equipment. A value of -0.31 or lower means the water is undersaturated (and hungry), so the water will dissolve calcium wherever it can find it, and pull it into solution.

Four of the LSI’s six factors have a direct relationship with the index, and the other two factors (CYA and TDS) have an inverse relationship. If these four factors increase, so does the LSI:
Some sources make the case that alkalinity combines with calcium hardness to form scale...and while in some cases that’s correct (carbonate ions bind to calcium to form calcium carbonate, CaCO3) alkalinity itself is not as strong of a factor as pH, and maybe even water temperature. The primary LSI factor that drives calcium precipitation is a high pH, but the location of calcium precipitation is often driven by water temperature.
You will never see calcium carbonate scale on the floor of a pool unless the scale is everywhere. White marks on the bottom of a pool are rarely scale; they are usually uneven carbonation as a result of aggressive water. True scale forms in the highest-LSI places first. Those places are inside salt chlorine generators and heaters. But the first place people can see scale is usually at the waterline, where it is warmest. Remember, higher temperature means higher LSI. Hotter water makes it easier for calcium to fall out of solution, plain and simple.

Importance of the LSI factors
Another comment about these four factors. Calcium hardness is much less of a driver of scale formation than many people think. For example, there is conventional wisdom in the pool business that you can prevent scale by keeping calcium hardness low. What they don’t realize is calcium hardness is necessary in the winter, and it offers stability for the LSI. Calcium hardness is the solid foundation to build your water chemistry strategy on. It’s so important, calcium management is our First Pillar of proactive pool care.
Related: The most misunderstood chemistry in the pool business
Now let's look at specific parts of the pool where scale is likely to form, and explain why.
Scale in salt chlorine generators

First, it helps to know the basics of how a salt chlorine generator works. Yes, saltwater pools are chlorine pools. They generate chlorine gas (Cl2⇡) on site using electricity to recharge chloride ions in the water. The other byproducts of this reaction are Hydrogen gas bubbles (H2⇡), Sodium (Na+), Hydroxide (OH-), and heat. See the graphic below.

Within the salt cell, the anode creates chlorine gas, while the cathode creates hydrogen gas, sodium, and hydroxide (these attract to each other to create sodium hydroxide, NaOH, a powerful base also called caustic soda, or lye).
Salt-generated chlorine is pH neutral
There is a common misunderstanding in pool care about why the pH rises in a saltwater pool. Most are taught that it's because the high pH of sodium hydroxide (NaOH) raises the pH of the pool. But that's technically not true when all the reactions complete themselves. In short, the high-pH Hydroxide ion (OH-) is neutralized by the low-pH Hydrochloric acid (HCl) produced when chlorine gas dissolves into water:
Cl2⇡ + H2O → HOCl + HCl
chlorine gas + water → hypochlorous acid + hydrochloric acid
Within several feet of plumbing after the salt cell, the HCl and OH- neutralize each other, so the chlorine that reaches the pool has a neutral pH. Yet the pH rises anyway. Why?
The pH in a saltwater pool rises thanks to physics, not chemistry. It turns out that the turbulence created by the Hydrogen gas bubbles (H2⇡) releases carbon dioxide from the water, and that raises the pH. We explain this more in-depth in another article here. Hydrogen gas bubbles are not very soluble in water (unlike chlorine gas, which dissolves very quickly and easily). These bubbles encourage the loss of carbon dioxide, thereby raising the pH.

If the LSI of the water is not balanced, and/or the pH ceiling is too high, the salt cell is likely to have calcium carbonate scale. And when the electricity changes direction in the cell (called reversing polarity), the acidic chlorine gas now dissolves the scale, causing it to fracture off into white flakes that blow into the pool or spa. This is a VERY common problem in saltwater pools.
Related: Calcium Flakes in Saltwater Pools
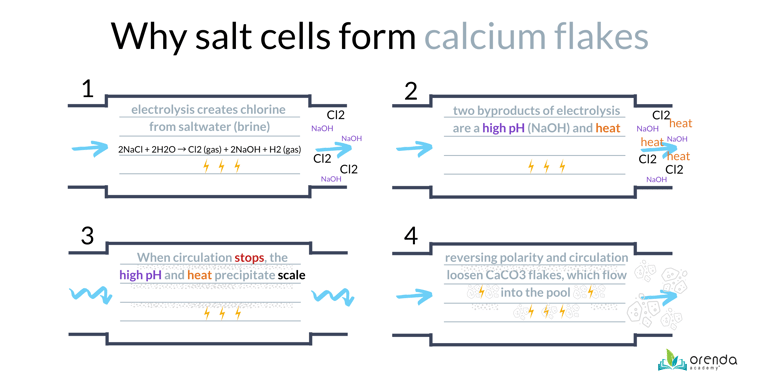
How to prevent scale in a salt chlorine generator
So how do we prevent calcium flakes in salt pools? There are just three things to do.
- Balance the water so that the LSI is balanced when the pH reaches its ceiling,
- Chelate the calcium with SC-1000 scale inhibitor, and
- Dial back the salt cell and/or program a cool down period where water flows slowly through the cell and flushes it out.
Let's explore each.
Balance the water

Instead of focusing on the pH right now, focus on where the pH is going. On the Orenda Calculator™, we display the pH ceiling, which tells us exactly how high the pH will naturally rise on its own. And since salt cells increase pH via physics (loss of CO2), we know the pH cannot exceed its ceiling.1
To balance the water properly for a saltwater pool, the LSI must be balanced when the pH reaches its ceiling.2
Applying this to the real world, when the water is warm enough for the salt cell to produce chlorine (around 60-65ºF for most salt systems), consider a lower total alkalinity level (around 60 ppm). This will reduce the pH ceiling to a more favorable level, making LSI-balance when the pH rises to that ceiling more feasible.
Chelate the calcium with SC-1000
.png?width=760&height=428&name=SC-1000%20(1).png)
If your water is properly balanced as just described, this step may not be necessary. It is still best practice to chelate metals and minerals in the water to prevent issues, however. SC-1000 is a long-term chelating agent that keeps calcium in solution, inhibiting scale formation. If the calcium in your water is chelated, it is spoken for, and unlikely to form scale in your salt cell (or anywhere else).
Cool down the salt cell
Most scale forms when the water is not circulating. The salt cell is still warm, and the water is stagnant. That's a high-LSI violation that gives calcium carbonate more time to precipitate and form scale.
To mitigate this, try to program a "cool down" cycle for your cell each day. With variable-speed pumps, this can be done by dialing down the pump to be moving water slower than the pressure-flow switch in the salt cell that engages the cell. For several manufacturers, water flowing slower than 25 gallons-per-minute will not have enough force to push the flow switch all the way on. So water slowly moves through the cell (flushing it out) while it is not on. It's a win-win.
You can also speak to your equipment manufacturers and service professionals about scheduling the salt system to shut off a few minutes before the pump does. But nowadays with variable-speed pumps, more and more pools are running 24/7. So consider option 1.

Snowflaking is just carbonate scale that breaks off the salt cell and flows into the pool
So far, every single case of calcium flakes in saltwater pools has been calcium carbonate (CaCO3). And that's a good thing, because with LSI balance and SC-1000, it's preventable and easy to clean up. However, many people in the pool industry have been told these white flakes are actually a different form a calcium scale called calcium phosphate. Thankfully, as far as we can tell, that is not the case.
Calcium Phosphate Scale

Occasionally, when there are high phosphate levels in the water, a denser type of scale known as calcium phosphate can develop in salt chlorine generators. This issue is particularly challenging to resolve and often necessitates equipment replacement. Fortunately, we have not yet discovered a salt system with calcium phosphate scale. All the flakes we have observed have been calcium carbonate.
We discuss calcium phosphate more in-depth in another article here. Calcium phosphate scale is easily prevented by:
- keeping phosphate levels low, ideally below 500 ppb (PR-10,000 and CV-700 can help), and
- chelating calcium with SC-1000. Chelated calcium will not bind to phosphate in the first place.
Related: Phosphate Removal (Pillar 3)
Scale in Pool Heaters

Heaters (or heat exchangers) transfer heat between coils and the water flowing through them. This transfer of heat means the water circulating through the heater is rapidly changing its LSI properties. This process happens fast, and there is usually not enough time to precipitate calcium carbonate if the LSI balanced in the pool.3
Here again, just like with chlorine generators, the problem is usually not when water is flowing. Scale is more likely to precipitate when circulation is shut off and the heater is still warm. The easy remedy is the same as before: shut off the heater at least 10 minutes prior to shutting off circulation. Allow the flowing water to cool down the heater more rapidly, and you can help avoid scale formation in it.
There have been samples taken from heaters of all three types of scale mentioned in this article: calcium carbonate, calcium sulfate and calcium phosphate. In fact, one heater had all three types of scale layered within its pipes. Remember that high temperature makes calcium less soluble, no matter what ion pair it is part of. And while the LSI does not predict calcium sulfate or calcium phosphate, temperature is still a factor in all three.
Preventing any type of scale in heaters should be accomplished by following the steps outlined in the previous section about preventing scale in salt cells. A heater presents a similar scenario to a salt cell, except it does not change the chemistry. It just encourages a localized LSI-violation if your water was not already LSI-balanced at the pH ceiling. And again, you can use SC-1000 as an insurance policy for further protection.
Conclusion
Scale is just an overabundance of a given calcium compound that needs to be deposited some place when water can no longer hold it. If and when a calcium compound becomes oversaturated, it will tend to deposit in the hottest places first. That’s why taking the time to cool the salt cell and/or heater down makes so much sense. And it doesn’t cost anything.
We hope this article helps you prevent scale in pool heaters and salt cells. If you have more questions, leave a comment below or contact us directly.
1 It would be different if salt cells were producing new hydroxides constantly...but they are not. They are recycling what is already in the water, and those hydroxides produced are neutralized by the hydrochloric acid (HCl) created when chlorine gas dissolves.
2 Use the Orenda Calculator™ and type in your values on the left side. Be sure to include water temperature, CYA and TDS too. When everything is in there, look at the pH ceiling value beneath the pH itself. That's telling you where the pH is going to rise to. While you're on the calculator, manually raise the pH for a moment, just so you can see what that future pH is going to do to your LSI. If you have a pH ceiling that is purple, there is a very high probability that the LSI will also be purple (+0.31 LSI or higher). This means calcium flakes are highly likely in that saltwater pool. On the right side, adjust the TA down to about 60 ppm to see what it does to the pH ceiling. If your pH at that new ceiling allows for a green LSI (0.00 to +0.30), calcium flakes are unlikely, and your pool will remain LSI-balanced thanks to being aligned with physics.
3 To expand on this, we are referring to the LSI of the pool itself, at the pool's temperature. The temperature inside the heater may be upwards of 140ºF when it is running. That, of course, is a large temperature difference (𝚫T) that could otherwise throw the LSI out of balance. But because water passes through the heater so quickly, there is not enough time for it to happen all at once. If it did, heaters would clog up quickly with calcium carbonate and water would not flow through at all, causing major problems. Instead, carbonate scale in heaters tends to happen gradually over time.
