Protect Pool Plaster with LSI Balance from the Start

Pool builders, plaster applicators, and service techs know that a pool startup can be a real pain. Plaster dust is just the tip of the iceberg. For residential pools especially, most pools have water filling them within hours of finishing the plaster. That means the tap water and its chemistry is immensely important in the curing process (hydration) of new pool plaster.
Covered in this article:
Aggressive Water vs. Cement
When water is below -0.30 on the Langelier Saturation Index (LSI), it will dissolve calcium carbonate into solution. This means that low-LSI water (aggressive water) will attack and etch cement, in the effort to balance itself on the LSI.
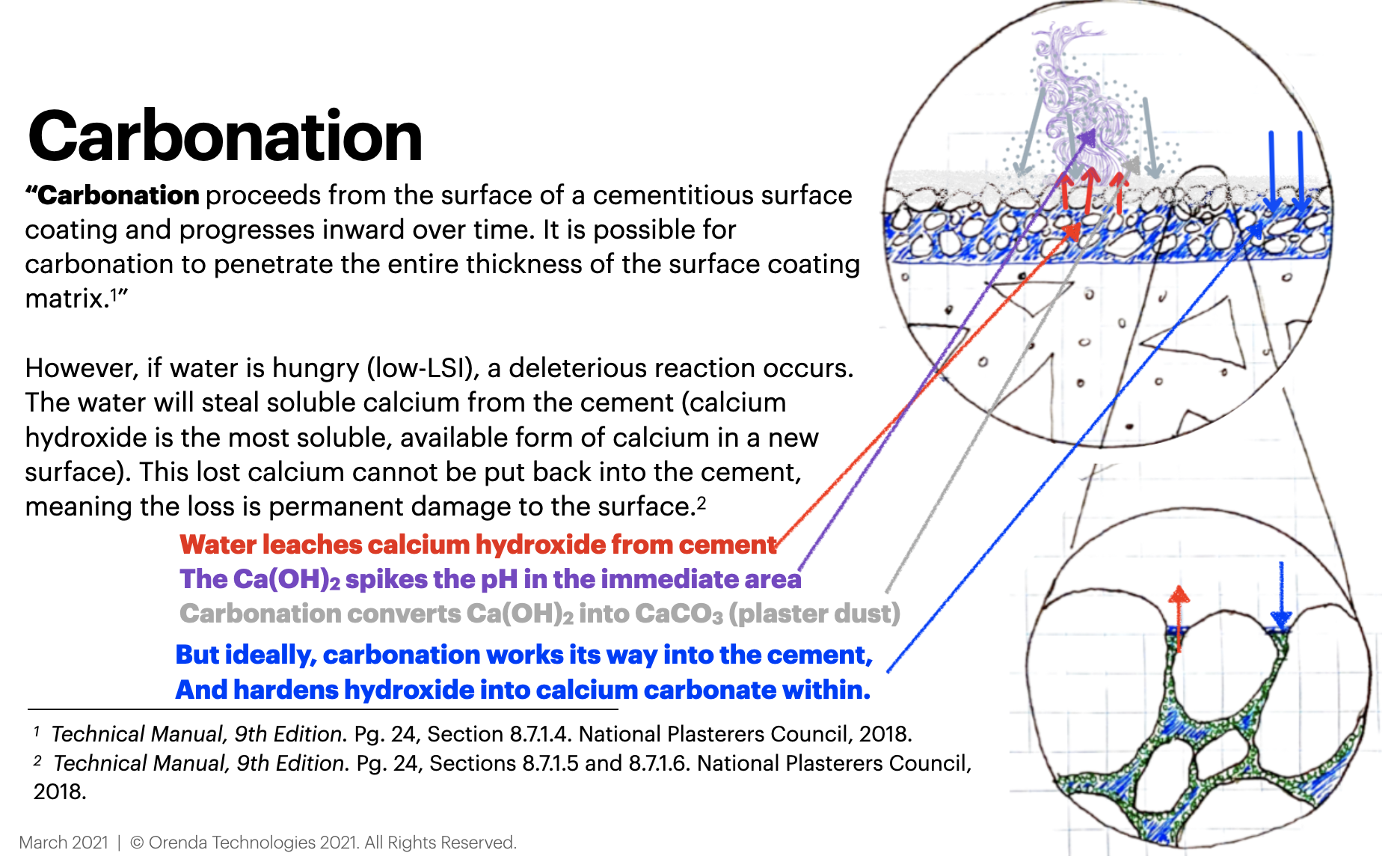
According to the National Plasterer’s Council (NPC) Technical Manual:1
"Acidic water aggressively attacks and dissolves cementitious surface materials. Water that is low in hardness, or soft water, can cause leaching of certain compounds of the cement, especially calcium hydroxide [Ca(OH)2]." - pg. 23
At Orenda, we believe–through undeniable field evidence–that urgency matters in water chemistry balance. We do not wait for the pool to be full to try and balance the LSI! We begin adjusting calcium as the water is filling unless there is 300+ ppm of calcium in the source water already (in which case we adjust alkalinity instead). In doing so, our customers and their pools have proven that plaster dust can be prevented entirely.
Related: The Orenda Startup™ is about the LSI. Not Calcium
The Orenda Startup™ is the result of smart, hard-working people in the field trying hard to prevent problems that almost always occur. Here's a video from Blue Moon Pools showing the difference between the pool being filled while adding calcium, vs. the spa that was filled without adding anything to it:
Risks of filling a pool with 'raw water'
Doing an LSI-focused startup can prevent many plaster problems, by preventing the loss of an important substance called Calcium Hydroxide (Ca(OH)2) from the cement as it cures. Calcium hydroxide is very basic (high pH of 12.6) and forms when water (H2O) mixes with Calcium Oxide (CaO) in the surface, in a process called hydration, which looks like this:
CaO + H2O → Ca(OH)2
Calcium Oxide + Water → Calcium Hydroxide
If you start up pools with cementitious finishes (like plaster, pebble, quartz, etc.), have you ever wondered why the pH is always off-the-charts high when the pool is full? And even after adding acid, when you return the next day, the pH is sky-high again? The pH spike during startup happens for a reason. Your cement is losing calcium hydroxide. So let's say you show up to the pool when it's full, and the pH is sky-high. Want to take a guess where the LSI is?
The LSI is perfect.
Because of its 12.6 pH, when it is drawn into the water, calcium hydroxide spikes the pH. The calcium hardness level also drifts up until the water finds 0.00 LSI on its own. The water then stops attacking the surface, because it has found its own LSI balance. But we, in an effort to comply with textbook range chemistry, see a super high pH and do what?
We were all taught to add acid.
Adding acid takes that perfectly-balanced water and makes it aggressive again. So the pattern repeats itself. Water gets hungry ("hangry" might be a better word to describe it) and takes more calcium hydroxide. Calcium drifts up again, and pH spikes again. We show up the next day and add acid again. The process repeats. Do you see how this goes? Our habits are actually making the problem worse. It's a self-fulfilling prophecy.
Test the fill water
This is why it is so paramount to test the fill water. We must know the water chemistry ahead of time so we can act accordingly. Test for water temperature, pH, total alkalinity, and calcium hardness especially.
And while we're talking about the fill water, measure it so you know the exact pool volume! If you don't know the volume–and write it down–the pool is doomed to inaccurate chemistry dosing for its entire life. The one shot that a pool has of having an accurate volume is if you use water meters while filling it, and/or know the volume of the trucks that fill it and do the math.
So, let's cover the risks of just filling the pool, disregarding the tap water chemistry. First of all, unless you are blessed with amazing tap water that is already LSI balanced, you will almost certainly lose some calcium. This loss of calcium will convert into plaster dust (which we will cover in a moment), and you will be brushing for days. It will also cost you a lot of acid to fight a constantly rising pH. The water chemistry will bounce up and down and will be difficult to nail down for at least a few days (and you will be spending money on all the pH and alkalinity adjustment chemicals like sodium bicarb and muriatic acid). But the real risks are long-term. Once calcium is stolen from the surface, it will not be put back. There are going to be microscopic cracks and voids in the cement, and the integrity of the surface will never be as strong as it could have been. This leads to many issues down the road, which is a conversation for another article entirely.
Low-LSI water causes Plaster Dust
Plaster dust is just carbonated calcium hydroxide. It forms when aggressive water steals calcium hydroxide from the surface–which is still trying to cure–and draws it into solution. As mentioned earlier, this happens so the water can find LSI balance on its own. So the pH rapidly rises near the surface, because Calcium Hydroxide's pH is an astounding 12.6, which is super basic. That high pH converts bicarbonate ions into carbonate ions (CO3) in the water. These carbonates and/or dissolved carbon dioxide (CO2) then bind to calcium and 'carbonate' it into calcium carbonate (CaCO3). The reaction looks like this:
Ca(OH)2 + CO2 → CaCO3 + H2O
Calcium Hydroxide + Carbon Dioxide → Calcium Carbonate (dust) + Water
This calcium carbonate looks like white dust, which we refer to as plaster dust. And it's a nuisance for pool startups. Most people think plaster dust is the problem; when in fact, plaster dust is merely a symptom of the real problem: the loss of calcium hydroxide. It is a permanent structural weakening of the surface, and almost always comes back to bite down the road. Here's a video explaining how plaster dust is formed:
Traditional Startup Methods
NPC 30-Day Startup
The industry standard startup protocol is the National Plasterers Council's 30-Day Startup. This process is loaded with great information, like not using wheeled vacuum cleaners (which can leave lines) and not adding salt until after 28 days. This startup card is a step-by-step guide for anyone to start up a pool, which makes it a valuable tool to pool builders and service techs in the field. But the NPC takes it a step further and serves the industry by offering startup certification courses. These courses are loaded with great information and are quite an education in themselves. Test kit do's and don'ts, the importance of testing tap water, and an understanding of how plaster hydrates and cures. We strongly recommend taking the NPC Startup Course if you have not already, even if you choose to do our Orenda Startup™. The knowledge and expertise shared in the NPC startup course is invaluable.
Hot starts
There is another old-school way to start up a pool called a hot start, or a zero alkalinity startup. In our opinion, it is NOT a viable startup. Rather, it is an exposure method that should not be done until at least 30 days into curing. Preferably longer.
A hot start basically gets the pH down so low (below 4.3) that there is zero alkalinity in the water. By getting below 4.3 pH, the pool's water takes longer to recover and reach LSI balance on its own. In the process, it dissolves calcium from the brand new surface. The idea is to make the pool look fresh and clean and even out the finish, especially after an ugly exposure or troweling job.
Hot starts often make the pool appear darker than it was originally, which, according to some sources, could be due to pigment oxidation. There are plenty of downsides to hot starts, and we at Orenda do not recommend them. We have an entire article devoted to hot starts and other exposure techniques for new plaster and pebble surfaces.
That being said, as a means of cleaning up a bad exposure process (like puddle rings, streaking, and other unsightly spots in the pool), a far less aggressive acid bath has a purpose. Rather than dropping the pH to zero alkalinity (below 4.3), this method takes the pH down to about 6.5 for a few days and serves as more of a cosmetic cleanup of the surface, rather than a 'scorch-the-earth' hot start. Hopefully, this isn't needed in your pool either, but it's a reliable option for cleaning up a messy surface.
LSI-based Startup Methods
The Orenda Startup™
 For startups, you can follow our Orenda Startup Guide, or watch some of our videos showing different ways to do it. You can also enroll in our free Orenda Startup Academy™ program.
For startups, you can follow our Orenda Startup Guide, or watch some of our videos showing different ways to do it. You can also enroll in our free Orenda Startup Academy™ program.
Download: The Orenda Startup 30-day Guide (PDF)
Our startup method is derived from a few facts that we learned from the NPC and industry experts about how plaster hydrates and cures, as well as how water chemistry interacts with a curing surface. The objective of our startup is simple: prevent the loss of calcium hydroxide.
Related: The Orenda Startup™ is about LSI, not just Calcium
We prevent the loss of calcium hydroxide from the start, by feeding the water the LSI saturation it craves, as the pool fills. The key is to get the LSI within the desired range (+0.20 to +0.30) as quickly as possible. Usually, that means adding calcium chloride (chelated with SC-1000) as the pool is filling, and sodium bicarb the next day.
Why do we need SC-1000? Because it allows for the rapid addition of calcium, as it is a scale inhibitor and chelating agent. Without SC-1000, adding calcium so quickly can cause calcium to fall out of solution and make a mess. The following day, we adjust the other factor (either alkalinity, or calcium, whichever one you did not add as the pool was filling), and purge the pool with CV-600 enzymes.
The Bicarb Startup
The Bicarb Startup was created by onBalance. Like our Orenda Startup process, the bicarb startup is focused on the LSI balance of the water filling the pool. There is no delay in adjusting chemistry. The entire point is to feed the water what it needs so it does not leach calcium hydroxide from the fresh cement. While we aim for an LSI of +0.20 to +0.30, the bicarb startup aims for about +0.50. And that works in their process because calcium hydroxide can cure in higher LSI environments faster. Since they are not adding calcium on day one, their risk of dusting is low.
The bicarb startup can be done in the Orenda startup barrel, and is essentially the same process, except dissolving sodium bicarbonate in the barrel instead of calcium chloride. Follow their process to learn more. If there is more than 250 ppm calcium hardness in tap water, we often advise customers to consider the bicarb startup using our same barrel. Because at the end of the day, the startup is about LSI balance, not how you get there.
Conclusion
Either give water the LSI balance it craves, or it will find balance on its own. The first several weeks are the most vulnerable for plaster, so protecting it while it cures is essential for the lifespan of the surface. Plaster dust is a choice, and we choose to prevent it.
1 National Plasterers Council. (2018). Technical Manual (9th ed.)., §8.7, pp. 23-25.

