Understanding LSI: The Langelier Saturation Index

The Langelier Saturation Index (LSI) is a cornerstone of the Orenda program. The LSI is the unbiased measurement of water balance, as defined by calcium carbonate saturation. It determines if our water is aggressive/corrosive (low LSI), balanced, or scale-forming (high LSI). It sounds simple enough, but let's dive in and show you just how much it matters to other aspects of water chemistry.
Covered in this article:
- What is the Langelier Saturation Index?
- Water always seeks equilibrium
- Analogy: Sugar in water
- How to calculate the LSI
- The six factors of the LSI
- The LSI equation
- What the LSI teaches us
- Calcium hardness is your friend
- Changing the way the world thinks about water
- Conclusion
What is the Langelier Saturation Index?

The Langelier Saturation Index (LSI) is the unbiased measure of water balance. Most water treatment industries use the LSI, and so do we. In the 1930s, Dr. Wilfred Langelier developed his index to know if/when water becomes corrosive or scale-forming. In short, the LSI tells us how saturated our water is with calcium carbonate (CaCO3). Perfect saturation is 0.00 LSI, and the acceptable range is between -0.30 to +0.30 LSI.
If the LSI is -0.31 or below, water is aggressive because it is under-saturated with calcium carbonate. The water is hungry for more calcium, and will do anything to find it. Above +0.31, the water has too much dissolved CaCO3, so it begins to precipitate CaCO3 out. The result could be carbonate scale, plaster dust, or other forms of CaCO3.
Water always seeks equilibrium
Perfectly balanced water is zero (0.00) LSI. Being the universal solvent, water finds its own balance and equilibrium because it wants to be at 0.00 LSI. But water is satisfied within the acceptable range (-0.30 to +0.30). If the LSI is low enough (-0.31 or lower), water will dissolve calcium from the most available sources first. In pools, that means the cement in the plaster or pebble finish. In fiberglass or vinyl liner pools, permanent damage will occur over time, like fading.
If the LSI is high enough (+0.31 or higher), water has too much CaCO3 in it. Water must precipitate CaCO3 to get itself back under +0.30 LSI and into the balanced range. If water is in LSI equilibrium, neither etching nor scaling will happen. The goal is to maintain LSI-balanced water year-round so that neither etching nor scaling occurs.
Related: LSI Balance & Calcium Management (Pillar 1)
Water will stop at nothing to find equilibrium, but water cannot over-saturate itself. It will take only what it needs and nothing more. That said, other factors can drive the LSI over 0.00, like rising water temperature, or the natural rise in pH from CO2 off-gassing.
Analogy: Sugar in water

When you add sugar to your drink and stir it, it dissolves. Add more sugar, and it will dissolve too. But at some point, when you add too much sugar, what happens? It swirls around at the bottom of the glass, unable to dissolve. That's because you have exceeded the drink's saturation limit; it can no longer hold any more sugar.
Now replace "sugar" with "calcium carbonate". That's the LSI in a nutshell. And it's worth noting that, unlike sugar, CaCO3 is more soluble in cold water. This explains why colder water temperatures mean a lower LSI.
How to calculate the LSI
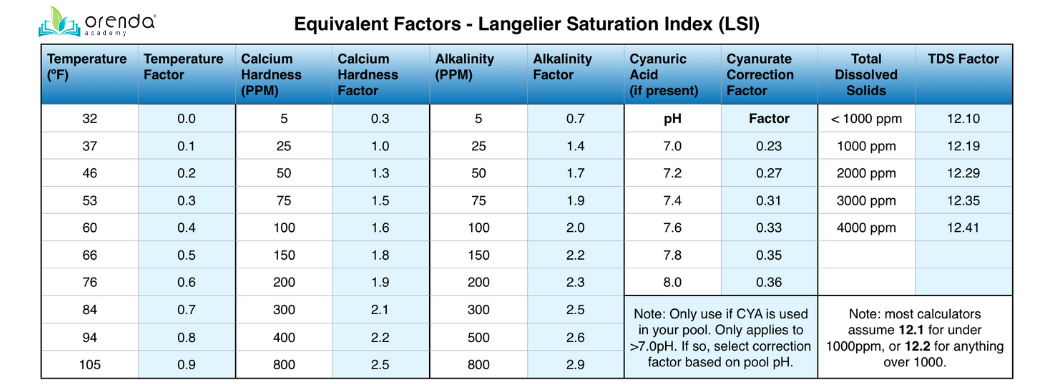
Six Factors of the LSI
The original LSI had five factors because it was made for boiler systems, not pools. But thanks to cyanuric acid, there are six variables you need to calculate the LSI for swimming pools:
-
Water Temperature (ºF)
Each parameter has a factor assigned to it–a numerical value that plugs into the formula. Before going any further, our free smartphone app, Orenda, does this math for you. So if you get lost in the details, that's okay! So did we. It's why we built the app. Now, onto the LSI factors. See the table below:
Each chemistry parameter has a factor assigned to it, except for pH. pH plugs directly into the LSI formula. The formula is simple enough, except when it comes to alkalinity. If CYA is in the pool, we must correct Total Alkalinity (TA) into Carbonate Alkalinity. This is because CYA contributes to TA as cyanurate alkalinity. To calculate the LSI, we must subtract Cyanurate Alkalinity from Total Alkalinity. Then use the Carbonate Alkalinity number for the alkalinity value in the table. We'll explain how to do this soon.
The LSI equation
In school, our math teachers required us to show our work when solving problems. So let's show how the LSI equation actually works. Here's the formula:
(pH) + (Temperature ºF factor) + (Calcium Hardness factor) + [(Total Alkalinity ppm) - (CYA ppm x correction factor @ current pH)] - (TDS factor) = LSI
In school, our math teachers required us to show our work when solving problems. So let's show how this formula actually works.
This is where things can get tricky. A few paragraphs ago we told you that the LSI calls for the carbonate alkalinity, not total. Carbonate (or "corrected") alkalinity does not include cyanurate. The formula has a correction factor for CYA to know the amount of cyanurate alkalinity. Multiply your CYA ppm by the correction factor, then deduct that from TA. That's your Carbonate alkalinity ppm. Then use THAT for your "Alkalinity" factor in the table.
- ph: (7.6)
- temperature: 84ºF (0.7)
- calcium hardness: 300 ppm (2.1)
- total alkalinity: 90 ppm (unknown factor until CYA correction is made)
- cyanuric acid: 60 ppm (pH 7.6 → [0.33 x 60] = 20 ppm cyanurate alkalinity)
- total dissolved solids: 1000 ppm (12.1)
Let’s calculate.
[(7.6) + (0.7) + (2.1) + [(90 TA) - (60 CYA ppm x 0.33)] - (12.1) = X LSI
(10.4) + [(90 - 20) alk factor] - (12.1) = X LSI
(10.4) + [70 alk factor] - (12.1) = X LSI
(10.4) + [1.7] - (12.1) = +0.00 LSI
On the Orenda App, we extrapolate within the LSI chart above, to get more accurate values between each factor. Therefore, the Orenda app shows nearly the same result as the chart, +0.02. This is nearly perfect LSI. The difference in this example is the alkalinity factor, which landed between 1.7 and 1.9...but where exactly? The formula by hand had to choose 1.7, but our app calculator was more precise:
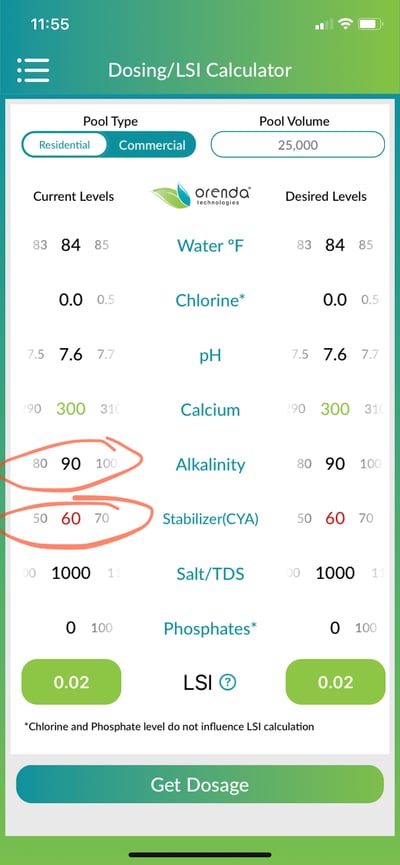
On the Orenda App, we get the same result, +0.02. But sometimes the Orenda App will show a slightly different LSI than your own math. This is because the table requires rounding up or down to the nearest factor. Our app is more precise because it extrapolates between factors.
We chose easy CYA and alkalinity values in the example above. But you won't always have 7.6 pH, which translates to exactly 0.33 (one-third) of your CYA to deduct. And you won't always have a CYA level that is evenly divisible by three (like 60 ppm). In most cases, you're rounding up or down throughout the formula, so results may vary.
We are often asked about the CYA correction and carbonate alkalinity. Again, the Orenda App makes this correction for you automatically. The correct way to use the calculator is to input your Total Alkalinity, CYA, and pH. You will know it's working when you adjust the CYA and your LSI changes.
What the LSI teaches us
The textbooks have taught us water chemistry ranges for decades. "pH should be 7.2 to 7.8, but ideally 7.4 to 7.6". Those are ranges. "Calcium should be 200-400 ppm." Another range. "Total alkalinity should be 80-120 ppm." Yet another range.
How you maintain the LSI may differ, but the end result should always be LSI balance. Michigan pools freeze in the winter and need much more calcium hardness than pools in Miami. Saltwater pools need less alkalinity and more calcium hardness than trichlor pools.
Calcium Hardness is your friend
The LSI teaches us the importance of calcium hardness. It is a consistent foundation for our water chemistry strategy. Parameters like pH and alkalinity fluctuate, but calcium does not. Also, calcium hardness itself is not the leading driver of scale formation. Just because scale is calcium carbonate does not mean your calcium hardness is too high. Scale occurs when the LSI is too high. Usually it's from high pH and high water temperature and alkalinity. Crazy, right?
Cold water needs more calcium hardness, but hot water does not necessarily need less. You could instead use less alkalinity, as long as the LSI stays balanced. We could go on and on with countless examples of different nuances, but hopefully you get the point. The LSI reigns supreme.
Related article: The Most Misunderstood Chemistry in the Pool Business
Changing the way the world thinks about water
Finally, the LSI offers a different way of thinking about water chemistry. Instead of focusing on maintaining chemistry ranges, (which can be physically impossible sometimes), focus instead on maintaining the LSI. Proactive pool care, anyone?