The CDC's Limit for Cyanuric Acid in Swimming Pools
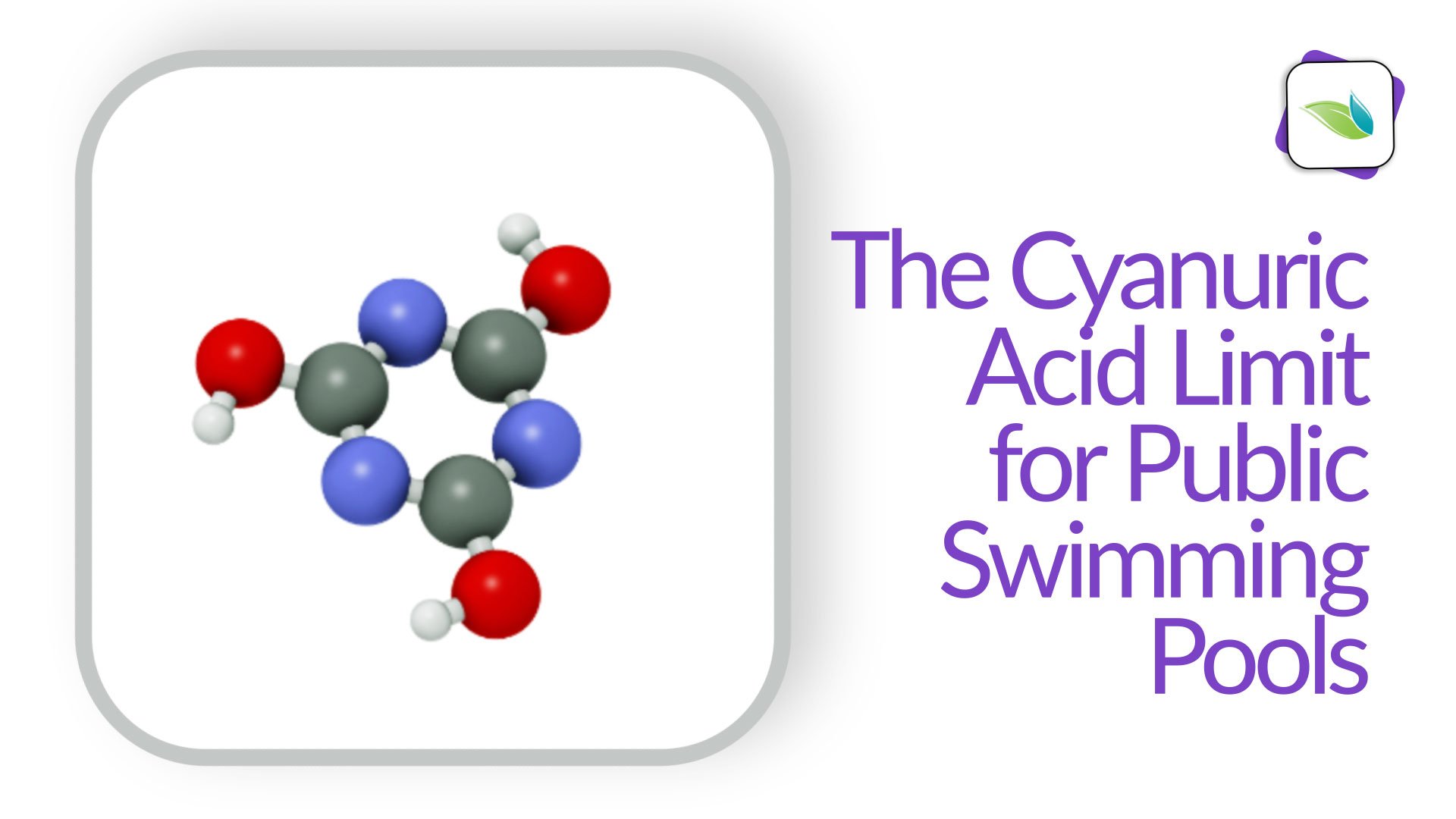
In the event of an Accidental Fecal Release (AFR), the CDC has published a recommended limit of Cyanuric Acid (CYA) at 15 ppm for commercial swimming pools. The vast majority of outdoor pools in America have far more than 15 ppm of CYA already, so what can be done?
Covered in this article:
- Why limit cyanuric acid (CYA) levels?
- Maximum CYA level for public swimming pools
- A new way of thinking about CYA
Why limit Cyanuric Acid (CYA) levels?
As discussed in several other Orenda articles, cyanuric acid (CYA) is a double-edged sword. It protects chlorine from sunlight, and at low levels, it is highly beneficial for outdoor chlorinated pools. But as CYA levels rise, their sunlight protection benefits plateau. The difference in sunlight protection between 30 and 70+ ppm is negligible.
However, higher levels of CYA are detrimental to both water quality and water balance.
For water quality, high CYA slows chlorine, which increases the contact times (CT values) required to kill harmful pathogens and algae.1,3 This reality is why the US Centers for Disease Control (CDC) and its Model Aquatic Health Code (MAHC) have published a limit to CYA for public pools. More on that in a moment.
For water balance, high CYA means more cyanurate alkalinity, which must be subtracted from total alkalinity when calculating the water's Langelier Saturation Index (LSI) balance. The more CYA, the lower the carbonate alkalinity and, therefore, the lower the LSI.
Related: LSI Balance and Calcium Management (Pillar 1)
Maximum CYA level for public swimming pools
The Council for the Model Aquatic Health Code (CMAHC) formed an ad-hoc committee for cyanuric acid, led by Dr. Chip Blatchley and Richard Falk. We often cite both in our articles. When speaking with Richard on the phone, he told us:
"The purpose of the suggested 15 ppm maximum for commercial pools was simply a practical matter. The contact times (CT) to kill RWI's like cryptosporidium become too high to be reasonably accomplished. So high that they were not worth measuring after a certain point, so we set a cap at 15 ppm for practical reasons, because the CT required was virtually impossible." - Richard Falk
Based on the research and findings of the CYA ad-hoc committee, the CDC published fecal incident response guidelines that state CYA levels cannot exceed 15 ppm.2 In our opinion, this is a good thing. It optimizes disinfection in public pools and also allows for enough CYA to protect most of the chlorine in the water from sunlight degradation.
Related: Minimal Cyanuric Acid (CYA) (Pillar 4)
A new way of thinking about CYA
In our opinion, this CDC guideline offers the swimming pool industry an opportunity to think differently about CYA. For decades, CYA has been misunderstood.4 Whether or not this CDC recommendation is enforced by local health departments remains to be seen. It will depend on the localities and their codes. But we think it's a positive thing.
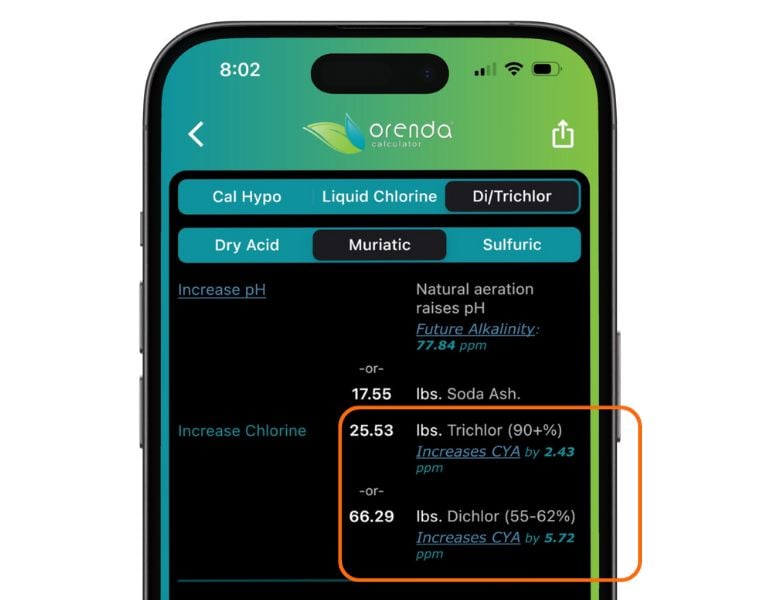
We at Orenda recommend minimal CYA on outdoor pools because we want sunlight protection without the baggage of overstabilization. This fits nicely into our Fourth Pillar of proactive pool care. And let's be honest, 15 ppm CYA is not difficult to achieve on a pool using liquid chlorine or cal hypo, because both are nonstabilized. It's the pools using trichlor as a primary chlorine that will be in violation of this rule, because trichlor (and dichlor) leave behind CYA as a byproduct. This CYA accumulates over time.
We now show the byproducts of chlorine types in the Orenda Calculator™, so you can see exactly how much CYA a given amount of trichlor or dichlor will leave behind in your pool.
1 In our article about reducing CYA, we cited several peer-reviewed studies about cyanuric acid's impact on chlorine and disinfection. If you want to read the science, scroll down to the footnotes and read the sources. The scientific evidence is clear: CYA slows chlorine down.
2 CDC (2018). Fecal Incident Response Recommendations for Aquatic Staff. Healthy Swimming. June 2018.
3 Falk, R.A., Blatchley, E.R., III, Kuechler, T.C., Meyer, E.M., Pickens, S.R., Suppes, L.M. (2019). Assessing the Impact of Cyanuric Acid on Bather’s Risk of Gastrointestinal Illness at Swimming Pools. Water. Volume 11, 1314.
4 We have customers who call and email us with questions about problem pools. Many of them do not know their CYA levels. Because CYA impacts both water balance and water quality, it is often part of the problems the customers bring to us. Issues like algae are directly correlated to higher levels of CYA because chlorine is slowed down. Most customers don't realize it. Another common misconception is that warmer temperatures require more CYA to hold chlorine for a week. But the truth is water temperature impacts how a pool uses chlorine, not how fast it loses chlorine to sunlight. Water temperature (and air temperature, for that matter) is unrelated to how well chlorine is protected from sunlight by CYA.
