Containing pH vs. Controlling pH in a Pool

The COVID-19 supply chain shortages have hit the swimming pool business. A parallel consequence of the chlorine shortage is now an acid shortage. This article will explain how to reduce acid demand by balancing your water chemistry in a more favorable way. You will learn how to stop chasing pH, and instead, learn how to contain it. The results will be less wasted chemicals and more predictable water chemistry.
Covered in this article:
What is Acid Demand?
The amount of acid your pool needs to maintain chemical balance is known as the acid demand. Acid demand is similar to chlorine demand, in that your pool is basically consuming the chemical. At Orenda, we have observed an industry-wide trend of overusing acid. In fact, abusing acid is the number one bad habit in our top 6 bad habits class. There are several reasons for this overuse of acid.
Related: What is pH?
Why is acid is overused?
First, traditional range chemistry parameters can lead pool owners and operators into trouble. Sure, textbook ranges can be good targets to aim for in the summer...but what about the spring, fall or winter? The fact is, trying to maintain certain chemistry parameters within the textbook ranges may be impossible during much of the year.1 In particular, trying to maintain a pH between 7.2 to 7.8, with an ideal of 7.4 to 7.6. If one is to maintain such pH levels, they either need to violate other chemistry range parameters (lower alkalinity, for example), or consistently treat with acid to combat the natural rise in pH. This is what we refer to as chasing pH. So why are we supposed to be maintaining these pH ranges in the first place? The books give three reasons: corrosion/scaling, bather comfort, and chlorine strength.
Corrosion and scale formation is not just pH, it's actually about the LSI. Of course, pH is a major factor in the LSI. But you can compensate for a lower pH with other factors, like more calcium hardness, carbonate alkalinity, or water temperature.
As for bather comfort, brands of bottled drinking water range from 5.5 to 9.5+ pH. That's a 10,000x difference in acidity, and yet we drink them just fine, and can hardly taste a difference. Are we really supposed to believe that it would irritate our skin more? Sure, at some point pH extremes can irritate skin and eyes...but in our opinion, you'd need to be outside of that 5.5 to 9.5 pH range. If your pool is outside those pH limits, you've got far bigger issues to worry about. Bather comfort is about chloramine byproducts...and chloramine in this sense is used generally to describe all disinfection byproducts. Bromine pools can have bromamines too. Basically, the byproducts of your sanitizer oxidizing nitrogen compounds and non-living organics can irritate skin and eyes. It's not the pH.
But the main reason is not bather comfort or corrosion/scaling. It's chlorine strength. So let's expand on that, as it is our second main reason acid is overused.
The pH is supposed to control the strength of chlorine like we have all been taught. And indeed it does, for an indoor pool, or any pool without any cyanuric acid (CYA) present. But when CYA is present, pH no longer dictates the strength of chlorine (%HOCl)! The free chlorine to cyanuric acid ratio does. The percentage of strong chlorine (hypochlorous acid, or HOCl) is virtually the same at 7.0 pH as it is at 8.5 pH when just 30 ppm of CYA is in the water. Here's the graph:

The chart on the left is what the textbooks still teach us. But most outdoor pools in America have at least some CYA in them. Look at the red line in both charts. See the difference? That's because when CYA is in the water, the vast majority of chlorine is bound to it. And pH does not dictate the strength of chlorine that is bound to CYA (called isocyanurates, represented by the purple line). So the argument that pH controls chlorine strength, at a practical level, only applies to non-stabilized pools.
The third reason acid is overused in pools is because acid is seen as the first (and easiest) option to remedy plaster discolorations and defects. Right or wrong, the habit has been to try acid to clear up plaster problems.
"Got scale? Add acid. Mottling and discoloration? Acid. Plaster dust? Acid. Trowel marks, streaks, and other major problems? Add a LOT of acid." - Bad Pool Industry Habits
But while acid has its purposes, it should usually be the last resort or next-to-last. Solving the LSI issue should be the priority. This can involve some diluted acid to adjust chemistry parameters but does not dump acid in the pool to burn away imperfections. There's a huge difference!
Controlling pH vs. Containing pH
Rather than trying to control pH, instead, try to contain it. By its very nature, pH moves around when you do anything to the water, and it naturally rises over time too. Trying to control it is futile–especially trying to control it within tight parameters like 7.4 to 7.6. In order to maintain a pH within that range, you have to fight against physics itself. Without an automated chemical controller and acid feeder, good luck with that.
Why pH naturally rises
We have another blog about what causes a high pH, and an even more specific blog about why pH naturally rises. To summarize, pH naturally rises in pools because pool water is more carbonated than the air above the water. This phenomenon is explained by Henry's Law of physics.2 In short, Henry's Law states that at a given temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas above the liquid. In other words, gas pressure will be in proportional equilibrium in and out of the water.
As it pertains to pH, the gas we care about is carbon dioxide (CO2). Why? Because while pH tells us the concentration of Hydrogen ions (H+), the amount of CO2 dissolved in water helps determine the pH of the water. The more CO2 dissolved, the lower the pH. The less CO2, the higher the pH. Here is a graphic from Robert Lowry to help visualize this:

Graphic by Robert W. Lowry. Used with permission.
Here's another analogy: a bottle of beer.
 In the bottle, a beer shows no bubbles. This is because CO2 has equalized within the bottle. But when the bottle is opened, pressure releases, and suddenly bubbles appear within the drink, rising to the surface. What you are witnessing is Henry's Law in action. CO2 is escaping the beer to equalize with the CO2 in the room. Eventually, equilibrium will be reached, and your beer will no longer be carbonated (it will be flat).
In the bottle, a beer shows no bubbles. This is because CO2 has equalized within the bottle. But when the bottle is opened, pressure releases, and suddenly bubbles appear within the drink, rising to the surface. What you are witnessing is Henry's Law in action. CO2 is escaping the beer to equalize with the CO2 in the room. Eventually, equilibrium will be reached, and your beer will no longer be carbonated (it will be flat).
The exact same physics applies to swimming pools too. We just cannot see the bubbles and the surface area of the pool is much larger.
So pH naturally rises because CO2 must escape the water and equalize with the air. Knowing this, how exactly are we supposed to control pH? Just to remind you, this is a law of physics we're talking about here...not a suggestion. The only way to really control pH, as mentioned earlier, is with chemical automation–either an acid feeder or CO2 injector, or both. In either case, you're trying to force pH to be somewhere it is not supposed to be. When CO2 equilibrium is reached, the pH cannot naturally rise any higher. We call this the pH ceiling, and given certain temperatures, your water's carbonate alkalinity determines this ceiling:
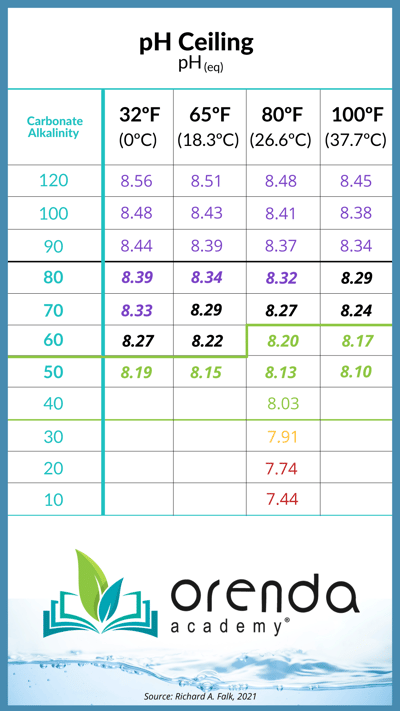
How to contain pH
Since controlling pH is a futile goal, let's instead contain it. Containment is actually pretty simple with the help of the Orenda LSI Calculator. Adjust your alkalinity to establish a pH ceiling you can manage.3 Then adjust calcium hardness high enough that at your new alkalinity target, your LSI remains balanced between the lowest desired pH, and the pH ceiling (based on the chart above). It sounds more complicated than it actually is. Here's an example of a liquid chlorine pool containing pH the Orenda way:
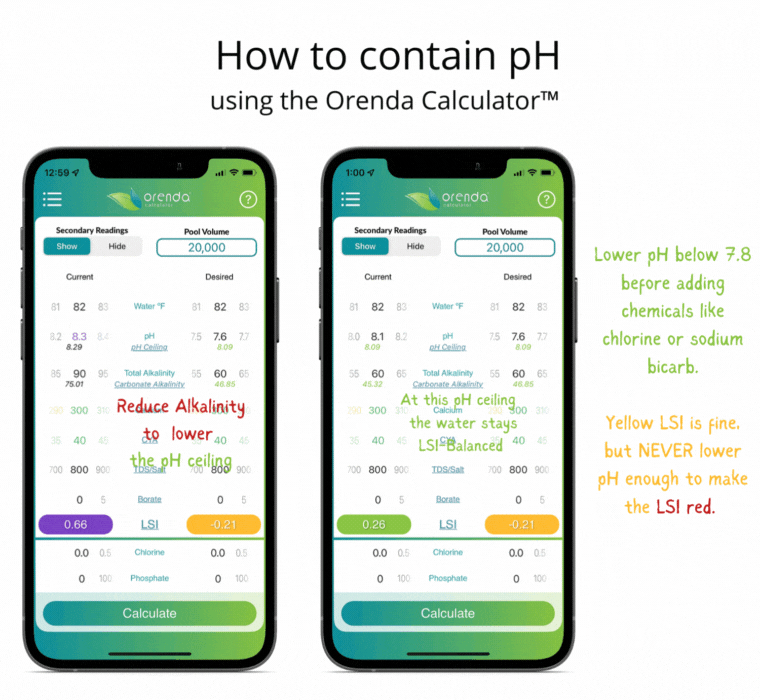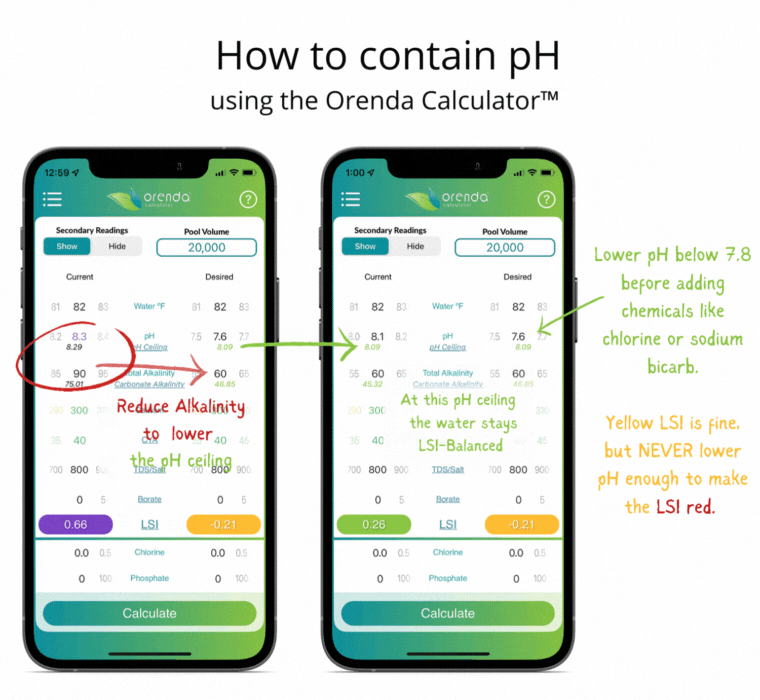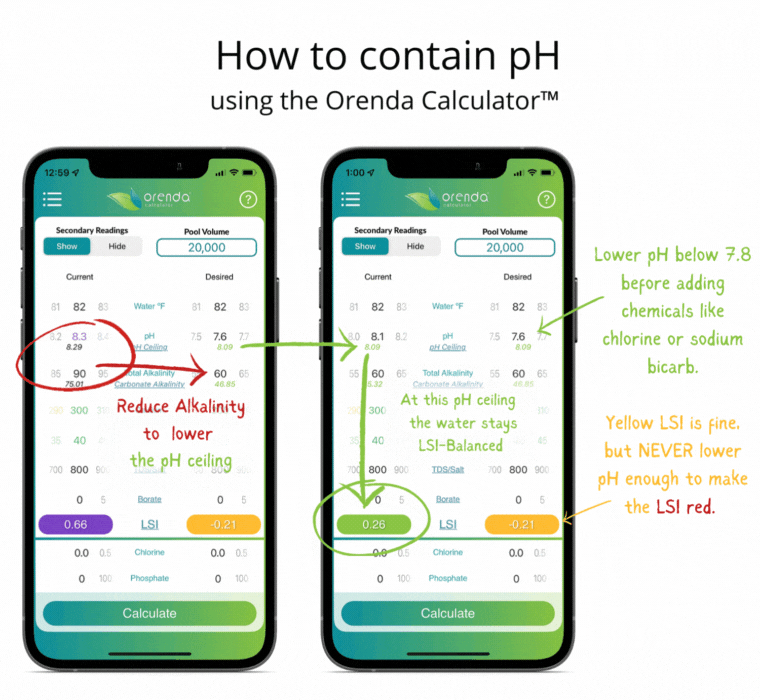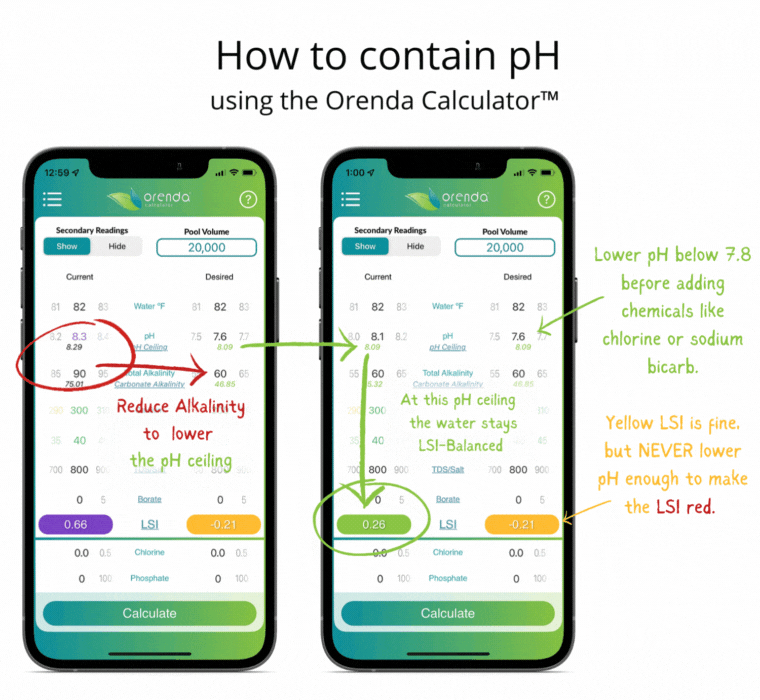 Notice the total alkalinity is less than the textbook-recommended 80-120 ppm. This is intentional so that when the pH climbs toward its ceiling, the LSI stays green. More alkalinity, at 8.0 pH, would cause scale. The only correction needed in this example is lowering the pH as low into the yellow LSI as we can go without it turning red. This threshold is what we call our LSI floor. So you have the LSI floor and the pH ceiling. These are the low and high of your contained pH. The parameters may change as the water temperature changes (mainly with calcium hardness), but the concept is the same regardless of temperature. The analogy we use is an elevator that contains your pH.
Notice the total alkalinity is less than the textbook-recommended 80-120 ppm. This is intentional so that when the pH climbs toward its ceiling, the LSI stays green. More alkalinity, at 8.0 pH, would cause scale. The only correction needed in this example is lowering the pH as low into the yellow LSI as we can go without it turning red. This threshold is what we call our LSI floor. So you have the LSI floor and the pH ceiling. These are the low and high of your contained pH. The parameters may change as the water temperature changes (mainly with calcium hardness), but the concept is the same regardless of temperature. The analogy we use is an elevator that contains your pH.
Related: Total Alkalinity vs. pH and their roles in water chemistry
Squared slowing of CO2 off-gassing and pH rise
There is another important thing to know about CO2 off-gassing. The closer your water gets to CO2 equilibrium (pH ceiling), the exponentially slower CO2 off-gases. It's a squared function. So while it might take a few minutes for pH to naturally rise from 7.2 to 7.3, it could take days for it to rise from 7.9 to 8.0. Left alone, this means water may never actually reach its pH ceiling in a week. If you have a salt system it might, but liquid chlorine and cal hypo pools may not. There are more factors at play. Just know that the speed of natural pH rise slows down at the rate of a square root.
The calculator screenshot above has taken this into account, showing the pH of only 8.0, instead of its more accurate ceiling between 8.13 and 8.20. Use this slowing of pH rise to your advantage. It gives you time, and makes the pH rise more predictable.
In essence, containing pH is accomplished by building your LSI strategy around a repeatedly rising pH. As long as you don't overcorrect the pH too low, your pH should rise at the same rate week after week, assuming the same alkalinity.
So containing pH is all about the LSI framework around the pH. Who cares what the pH is between its floor and ceiling? You know where the finish line is! And your chlorine's strength in a pool with CYA is not married to pH anymore. So let it rise because pH will rise anyway.
Ways to reduce acid demand
Compared to the traditional practice of trying to control (or chase) pH, containing pH our way can save you a tremendous amount of acid and sodium bicarb. Think about it. Your acid doses will be predictable, and because you have less alkalinity in the water, the doses will be smaller too. Furthermore, less acid being used means less alkalinity being neutralized, so you won't need as much bicarb either. You're making micro-adjustments, not major adjustments.
Apart from the containment strategy itself, here are a few other ways to save on acid:
- Always dilute acid. Not diluting means acid can get to the bottom of the pool and etch cement/plaster surfaces, which will neutralize the acid and cause an even higher pH after you leave.
- Always measure acid. No, your 20,000-gallon pool probably does NOT need a half gallon to make a pH correction. Know your alkalinity and pool volume, and the Orenda app will tell you exactly how much acid is needed. It's often less than you might think you need.
- If you're going to err with an acid dose, always err light. Never overdose acid.
- Know your tap water chemistry. If you have high alkalinity or high pH out of the tap, acid makes sense. If you have low alkalinity and low pH out of your tap, perhaps a CO2 system might be better for your pool. Just be aware of the algae issue with CO2 feeders on outdoor pools (algae feeds on CO2).
- If you have chemical automation, consider a timed feed of acid, rather than 'sense and dispense'. This means your pool will always get the exact amount of acid needed to get back to the desired pH, rather than feeding acid until the pH probe recognizes a change. Oh, and also, feed acid on the slowest feed rate possible to maximize dilution. If you're a commercial operator struggling to maintain pH and alkalinity, this is worth a longer conversation with us. Request a private training session here.
- Change the habit of 16 oz. of muriatic acid per gallon of liquid chlorine. You don't need that much acid, because technically liquid chlorine only temporarily raises pH. Once it kills or oxidizes, the pH comes back down because chlorine releases an almost-equal amount of muriatic acid (HCl) to neutralize itself. That said, a little bit of acid can help take the edge off shortly before or after adding liquid chlorine or cal hypo. Instead of 16 oz. of acid, try 8 oz, or even 4 oz.
- Stop using acid as a first resort when it comes to plaster issues. Test parameters and adjust the LSI. Yes, LSI adjustment may involve diluted acid to make a pH or alkalinity adjustment, but that's not the same thing as dumping acid in a pool to clean up a surface. Such heavy acid use may have its place, but it's not the first option.
Conclusion
We overuse acid in the pool business. Now that acid and chlorine are facing supply chain shortages, we all have an opportunity to think differently about how we treat swimming pools. The traditional way to maintain a pool is following textbook ranges, like trying to maintain a pH between 7.2 and 7.8. But if you have CYA in your pool, we argue that is no longer necessary. pH still matters of course, but we should contain pH rather than trying to control it. Once we come to accept the fact that pH will naturally rise due to CO2 equilibrium (Henry's Law), we can choose to not be tied to such narrow pH parameters. Generally, you want to stay between 7.5 and 8.0, though most pools will have a pH ceiling around 8.1 or 8.2. This article covered how to lower that ceiling by simply lowering carbonate alkalinity, and then adjusting other LSI parameters to maintain balance with that lower alkalinity level.
This article is not conjecture and hypothetical ideas. It works, and we have proven it time and again. Obey physics and water will behave predictably. Beyond simplifying your maintenance routine, you will save big on acid and sodium bicarb, because you are no longer chasing pH. Stop wasting money and chemicals trying to force water to maintain a pH it cannot maintain. Instead, embrace physics, contain pH, and let it rise each week before resetting it.
The amount of acid you save might surprise you.
1 This is why we teach LSI first, range chemistry second.
2 Chemistry LibreTexts. Henry's Law. Updated February 2021.
3 Adjust your total alkalinity (TA) in the app, but the pH ceiling is based on carbonate alkalinity. When implementing this containment strategy, take one-third of your cyanuric acid, and deduct that from your TA (0.33 x CYA).

