Pool Sanitizers, Part 2: Chlorine Alternatives

While chlorine is the most commonly-used residual sanitizer in the world, there are some alternatives. But not every chlorine alternative is capable of doing everything chlorine does, nor is it capable of doing the job as well as chlorine does. Free chlorine is the most popular sanitizer for a reason. That said, here are some non-chlorine alternative sanitizers.
Covered in this article:
Chlorine alternatives (overview)
In part 1, we discussed the various chlorine products on the market and why a residual pool sanitizer is essential for a safe, clean, and clear swimming pool. Now, let's take a look at chlorine alternatives.
Bromine
- Byproducts in pool water: hypobromous acid (HOBr) + hydrobromic acid (HBr)
- pH: Bromine is 3.8-4.0 (strongly acidic), and Sodium Bromide is between 6.5 and 8.0.
Pool operators can introduce bromine as sodium bromide (bromine "salt"), with the addition of another oxidizer like potassium monopersulfate (which we will cover next). Sodium bromide is most commonly used as an algaecide, though we strongly advise against doing so.1
Bromine is usually used in hot tubs and spas as bromine tablets. There are two types of bromine tablets:
- BCDMH (bromo-chloro-5,5-dimethylhydantoin) tablets or sticks
- 4.5-4.8 pH (moderately acidic due to the HOCl created)
- Chemical formula: C5H6BrClN2O2
- Contains some chlorine, and both HOBr and HOCl will be created
- Most popular bromine product on the market, specifically for spas.
- Does not require a separate oxidizer to work, though beginning with sodium bromide to build a "bromide bank" in the water is recommended when first using this product
- DBDMH (dibromo-5,5-dimethylhydantoin) tablets or briquettes
- ~6.6 pH (relatively neutral)
- Chemical formula: C5H6Br2N2O2
- Chlorine-free
- Also requires a separate oxidizer to oxidize bromide ions into active bromine
These tablets can be added to a floater or an inline bromine feeder, similar to a cal hypo feeder. Like chlorine, bromine will not disinfect until the oxidant demand has been destroyed.
Related: What is bromine?
Pros: Bromine in swimming pools behaves differently than chlorine in a few ways. First, unlike chlorine, bromine can be recycled. After bromine is used up, the bromide ion (Br-) can be recharged via oxidation by a separate oxidizer. Oxidizing bromide converts it back into hypobromous acid (HOBr), the killing form of bromine. While it is weaker than the active form of chlorine (HOCl), HOBr is effective against algae, germs, and oxidants.
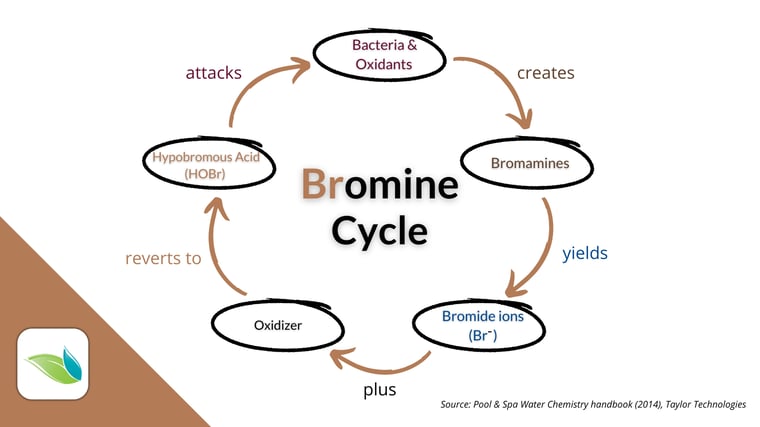
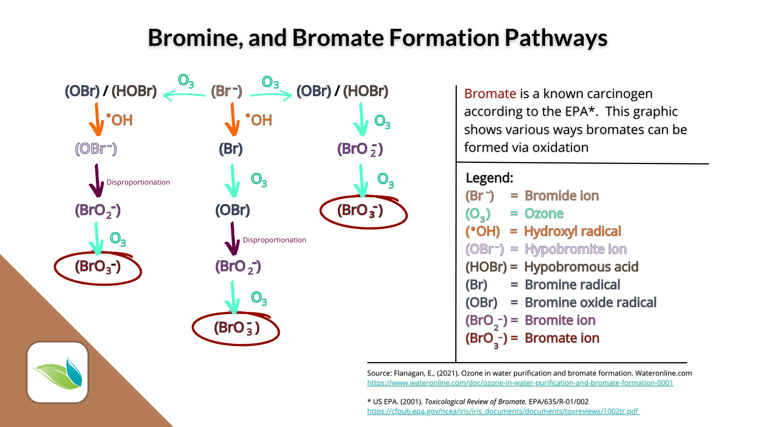
Cons: There is no sunlight-protection stabilizer for bromine. Therefore, HOBr breaks down in direct sunlight quickly, forming a byproduct the EPA considers carcinogenic: bromate ion (BrO3-). Therefore, bromine should NOT be used in outdoor pools.
There are limited options for compatible oxidizers that can recharge bromine without creating bromates. Chlorine and potassium monopersulfate are the only ones recommended in bromine pools, because Ozone and AOP systems will also create bromates. See the graphic below.
Potassium Monopersulfate (KMPS)
- Byproducts in pool water: Potassium, sulfates
- pH: 2.1 (extremely acidic)
Pool professionals use potassium monopersulfate (or potassium peroxymonopersulfate) (KMPS or MPS) as a non-chlorine oxidizer shock. It is compatible with chlorine pools and bromine pools. Because KMPS is not a sanitizer/disinfectant, it cannot stand on its own as a chlorine alternative. There still needs to be a primary residual disinfectant like HOCl or HOBr in the water.
Pros: KMPS boosts sanitizer efficiency because it helps oxidize contaminants. In that sense, using KMPS as a shock is another way to remove organic waste. It is effective at oxidizing nitrogen compounds prior to them combining with chlorine. This means KMPS is helpful for reducing chlorine demand during the breakpoint chlorination process, albeit indirectly.
Cons: Potassium monopersulfate tends to give some test kits a false reading for combined chlorine. This is due to how it reacts with reagents. KMPS is one of the more expensive chlorine alternatives, and it does not disinfect...it only oxidizes. And contrary to popular belief, potassium monopersulfate does not eliminate chloramines once they have been formed (meaning chlorine has already combined with the nitrogen compound).
Biguanide (PHMB)
- Organic nitrogen polymer (hydrocarbon). Formula: (C8H17N5)n
- Sanitizer, not an oxidizer. Requires a secondary oxidizer like Hydrogen Peroxide (H2O2).
Polyhexamethylene Biguanide (PHMB) is a cationic polymer with disinfection properties.2,3 This polymer is a synthetic organic substance that contains nitrogen, and it directly conflicts with chlorine and many other commonly used pool chemicals. This includes salt chlorine generators, mineral systems (including ionizers), certain sequestering agents, bromine, and copper algaecides.4
And since biguanide is only a sanitizer–not an oxidizer–a secondary oxidizer is required. Unfortunately, potassium monopersulfate and secondary oxidation systems like ozone are incompatible with biguanide. Biguanide pools are limited to using hydrogen peroxide (H2O2) as an oxidizing agent. Using potassium monopersulfate conflicts in an especially dramatic way, in that the water can turn orange and precipitate a gel-like substance.
Once a pool is using biguanides, it would need to be completely drained, rinsed out and refilled if the pool is ever to be converted into a chlorine pool.
Pros: Biguanides are less physically volatile than chlorine or bromine, and are regarded as relatively safe to handle. Biguanide also tends to last longer in the water than chlorine or bromine. The perception of having a "chlorine-free pool" is appealing to many homeowners, and using biguanide as the primary pool sanitizer can accomplish that. Biguanide is also gentle on skin, pool liners and bathing suits.
Cons: Biguanide products sanitize less efficiently than chlorine, given that it takes 30+ ppm to sanitize water (compare that with 1.0-3.0 ppm free chlorine). Biguanides are also expensive products. And since they do not oxidize, removing bather waste from the water remains a problem. Given that Biguanide is an organic polymer, it flocs particles and contaminants together, which is then captured through filtration. This leads to clogged filters over time, which means more regular filter cleaning. Perhaps the main downside of biguanides is its incompatibility with just about every other pool product on the market.
Metals

Many residential pools use dissolved heavy metals as a sanitizer, but not an oxidizer. Research shows that dissolved metals are more effective against algae than germs and viruses. Most metal products are in the form of an algaecide, either silver or copper-based. There are also mineral ionizers, which utilize electricity to introduce something like copper into the water at higher levels. These systems advertise a "chlorine-free pool".
Pros: Metal products are effective algaecides, and are widely available on the market. They are generally inexpensive and easy to use.
Cons: In our opinion, metals no replacement for a residual sanitizer like chlorine–especially since metals cannot oxidize non-living contaminants. Remember that 90+% of contaminants in water are oxidants...and metals cannot manage them. In fact, metals are part of the oxidant demand on chlorine:
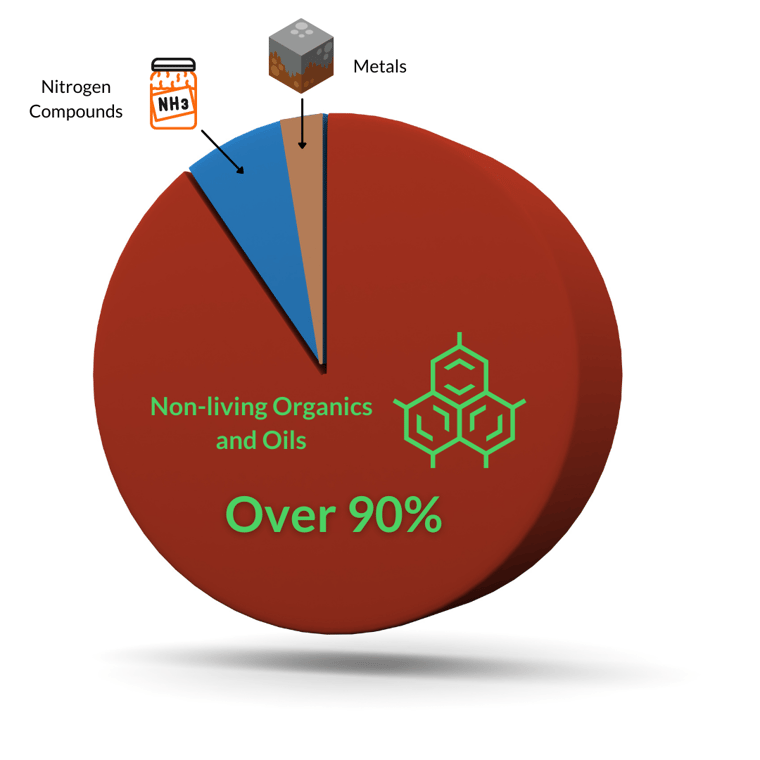
At a certain point, dissolved metals can be harmful to people, especially if accidentally consumed (yes, people accidentally swallow pool water). But, according to manufacturers of these products, the metals are chelated and therefore less of a risk. And there is some validity to that. These metal products are chelated with a substance called Ethylenediaminetetraacetic Acid (EDTA). EDTA is another hydrocarbon that chlorine will oxidize and break down, just like sunlight will.5,6
And even when using long-term metal-chelating products like SC-1000, metals can still over-saturate and cause problems. And at a high enough pH, those oversaturated metals can create stains.
We at Orenda are not proponents of using any heavy metals as chlorine alternatives in pools...even though they are effective sanitizers. We already have to deal with metals in the source water, so deliberately adding more to the pool just compounds the issue and leads to more problems (like stains). But more importantly, they stay in the water for a long time, which violates the Orenda philosophy.7
Secondary disinfection systems
Secondary disinfection systems are widely used in commercial pools and are gaining popularity in residential pools, too. These systems are not chlorine-based, and they are not meant to replace chlorine. Secondary sanitizer systems are designed to supplement chlorine against harmful pathogens. The powerful oxidizers Ozone (O3) and Hydroxyl Radicals (•OH) also supplement chlorine against the oxidant demand.
All of these systems are secondary–not primary–because they do not create a residual in the pool itself. They are all point-of-contact systems.
Ultraviolet (UV)
The most popular secondary disinfectant, by far, is UV. UV is available in both medium-pressure and low-pressure systems. These refer to wavelengths of light that inactivate a specific array of pathogens. UV is effective at destroying formed chloramines...but not their nitrogen precursors (because UV is not an oxidizer). It inactivates DNA/RNA of living pathogens so they cannot reproduce.
Ozone
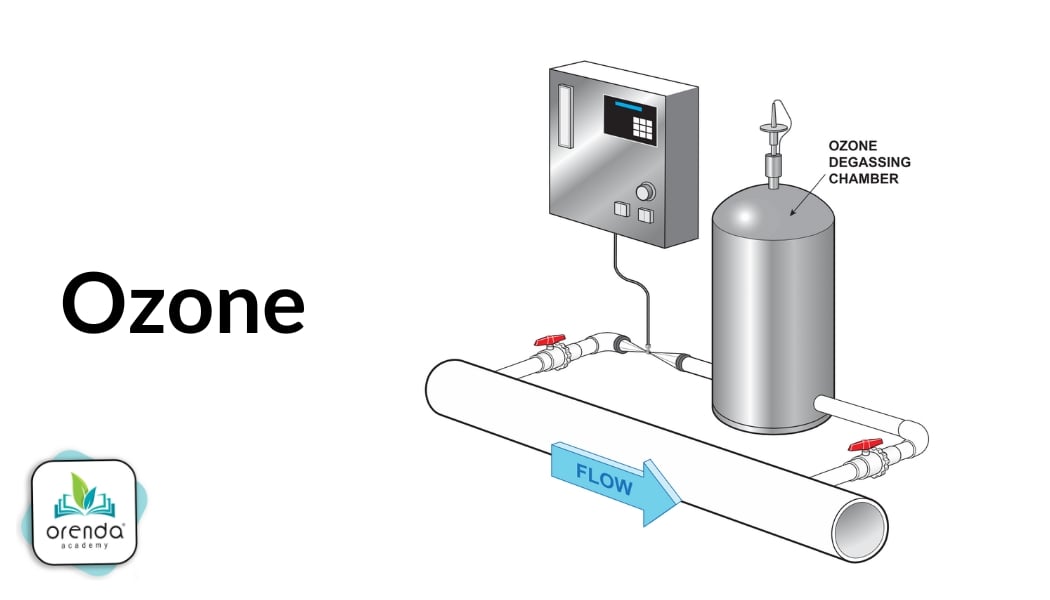
Ozone (O3) is and incredibly powerful oxidizer. It is created by splitting oxygen (O2) using a disruptive energy source, such as UV light, corona discharge, or microplasma. Oyxgen radicals (O) are unstable so they bind to the nearest O2, creating ozone (O3).
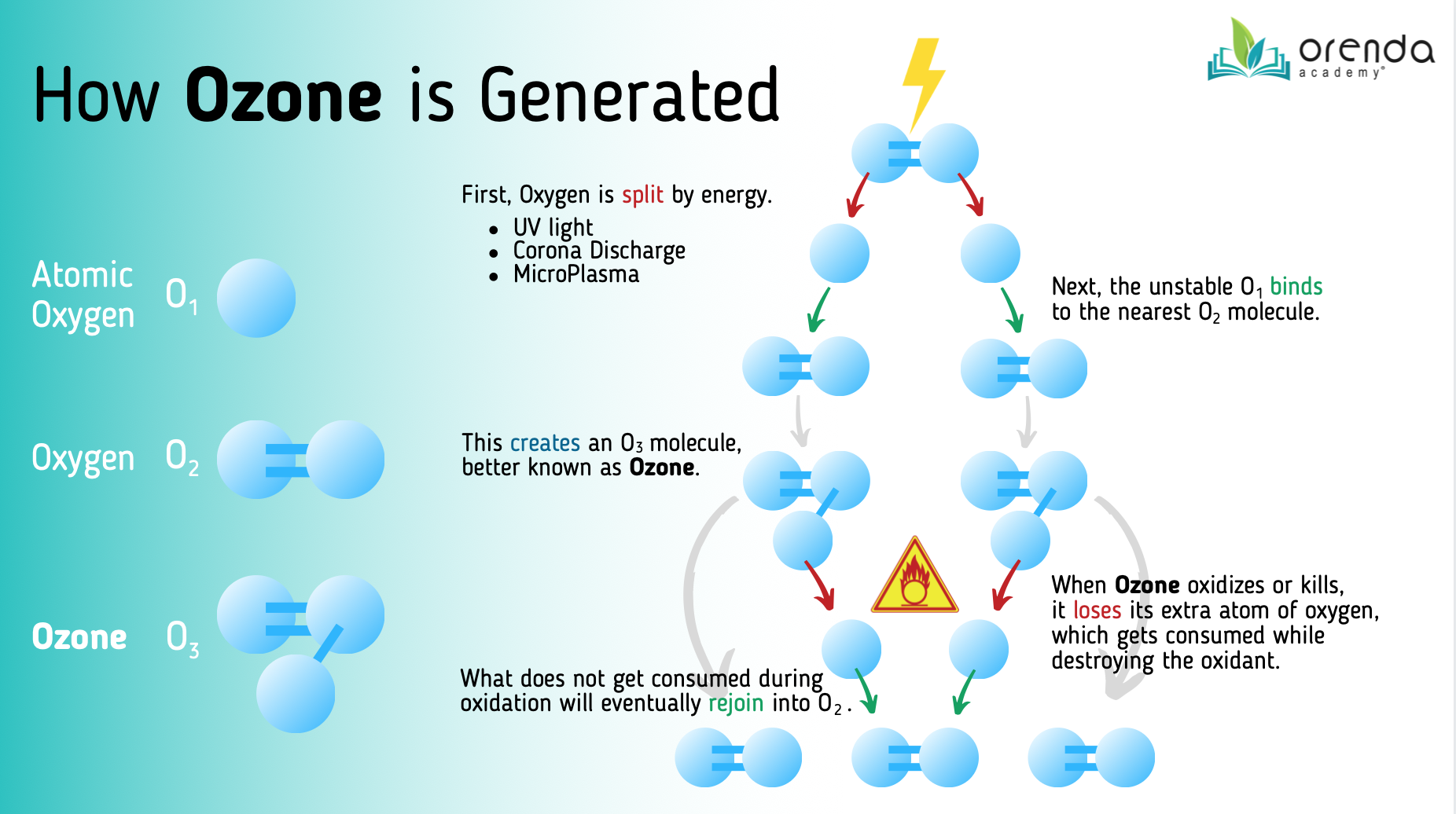
Ozone can kill and destroy just about anything in the water in a single pass, including chlorine-resistant parasites like Cryptosporidium.
AOP
Ozone is not the only powerful oxidizer available on the market. Advanced Oxidation Process (AOP) is a system that creates hydroxyl radicals (•OH). Hydroxyl radicals are even stronger oxidizers than ozone.
But with both of these systems, the question is how effectively can these gases be dissolved in water, and for how long can they remain in the water? Ozone and hydroxyl radicals only last for a few seconds in the water, which translates to 6-8 feet of plumbing (usually). But in those precious few seconds, any water passing through that pipe will be thoroughly oxidized and disinfected.
And that's the downside of these secondary systems. They are point-of-contact only; therefore they are incapable of fully replacing chlorine.
Conclusion
With time, we expect to see new emerging technologies and products available for use in swimming pools. Until a reliable alternative to chlorine can create both residual disinfection and oxidation, chlorine is here to stay. We encourage you to continue to do more homework on this if you are looking for the best options for your pool.
1 There are several long-term consequences of using sodium bromide in water. Especially in outdoor swimming pools. You can learn more in our bromine article or by listening to episode 114 of our Rule Your Pool podcast. The Orenda philosophy is 1) proactive pool care, with 2) no chemical conflicts, and 3) no long-term byproducts left behind. Sodium bromide is not proactive, it directly consumes chlorine (and therefore conflicts with it), and it stays in the water indefinitely. The bromine cycle means chlorine continues to oxidize bromide ions into HOBr, which cannot be protected from sunlight degradation, so it does not last long. And to make matters worse, direct sunlight converts bromine into the harmful bromate ion (BrO3-). So again, we strongly advise against using sodium bromide in swimming pools.
2 Sowlati-Hashjin, S., Carbone, P., Karttunen, M. (2020). Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. Journal of Physical Chemistry. 124(22), 4487–4497.
3 Asiedu-Gyekye, I. J., Mahmood, A. S., Awortwe, C., & Nyarko, A. K. (2015). Toxicological assessment of polyhexamethylene biguanide for water treatment. Interdisciplinary Toxicology, 8(4), 193-202.
4 Taylor Technologies: The Lowdown on a High-Ticket Sanitizer: Biguanide.
5 EDTA is widely used in the medical field to address heavy metal toxicity, such as lead poisoning (it's called chelation therapy). In swimming pools, however, EDTA is a chelating agent to bind with metals and minerals to prevent oxidation and staining. The only issue is it is a hydrocarbon that breaks down in chlorinated water and direct sunlight. EDTA's formula is [CH₂N(CH₂CO₂H)₂]₂. So, over time, EDTA releases the metals so they can again be oxidized and cause staining in the pool. Our chelating agent, SC-1000, is not EDTA, and it is immune to chlorine oxidation and sunlight degradation.
6 George, T., Brady, M. (2023). Ethylenediaminetetraacedic Acid (EDTA). Stat Pearls. Creighton University School of Medicine.
7 Much like sodium bromide, which we described in our first footnote (1), metal systems violate our Orenda philosophy too. Metals conflict with chlorine, and they stay behind in the water long-term. If you have metals in your water, we strongly suggest doing what you can to remove them. They only make pool care more complex.
