Understanding Calcium Carbonate Scale

Carbonate Scale is a buildup of hardened calcium carbonate (CaCO3) on pool surfaces or equipment. Scale can be a big problem for a pool and its plumbing system (and other water systems besides pools, like fountains).
Covered in this article:
- What is scale?
- What is NOT scale?
- Calcium chemistry
- Calcium carbonate saturation and the LSI
- How to remove and prevent scale
- Swimming pool scale removal
- Swimming pool scale prevention
- Other types of scale (besides calcium carbonate)
- Calcium phosphate scale
- Calcium silica scale
- Calcium sulfate scale (crystals)
- Conclusion
What is scale?
The term "scale" is used to describe a hardened mineral buildup on surfaces, equipment and pipes of a water system. Unfortunately, most definitions online refer only to calcium carbonate scale, also known as limescale. As we will discuss later in this article, there are several other forms of scale that are not carbonate scale.
Here's our own definition of scale:
Scale is a buildup of mineral compounds (usually calcium-based) that have precipitated out of solution and have formed on surfaces in and around the water system.
Let's expand upon this definition so we clearly define what we're talking about.
Scale is a buildup of mineral compounds. Minerals refer to alkali metals and alkali earth metals. These are groups 1 and 2 of the periodic table of elements.1 There are 12 such elements, but when it comes to scale formation in water, we usually only deal with two alkali earth metals: magnesium and calcium. And of those two, 99% of the scale you will find in water is a calcium-based substance. So let's stay focused on calcium.
...that have precipitated out of solution. This is the defining feature of scale. Scale always originates from an oversaturation in the water. If the calcium compound (usually CaCO3) is oversaturated (above +0.30 on the LSI in the case of CaCO3), the water will get rid of some of it to return itself to equilibrium. The analogy of sugar in a drink explains this well. If your drink has too much sugar in it, sugar will sink to the bottom no matter how hard you stir. But simply heating up your drink may allow for that sugar to dissolve. The saturation point of the drink changed when the temperature did. Scale is when your water has too much of a given calcium compound in it, so it has to get rid of some of it.
...and have formed on surfaces in and around the water system. Because scale comes from the water and falls out of solution, it lands on surfaces regardless of their material type (i.e. plastic fittings, metal ladders, tile, lights, etc.). Calcium carbonate scale, in particular, always forms in the highest LSI areas first. Usually, that means the warmest water and the highest pH. The point is that scale lands on any type of surface, so if you only see calcification on cement and nothing around it, it's not scale.
What is NOT scale?
A common mistake pool professionals make is thinking that any and all calcium deposits are scale. That's simply not true. Just because acid cleans it up does not mean it was scale in the first place. It was likely calcium carbonate, yes, but calcium carbonate also presents itself in etching, mottling/discolorations of cement surfaces, plaster dust, winter dust, and calcite crystals.
In short, if the calcium did not originate from the water (due to oversaturation) and fall out of solution, it's not scale.
 This is NOT scale. This is from pouring undiluted acid around the perimeter of the pool. The acid dissolves the carbonate layer of the cement, drawing calcium hydroxide to the surface, which then converts into calcium carbonate and turns pure white.
This is NOT scale. This is from pouring undiluted acid around the perimeter of the pool. The acid dissolves the carbonate layer of the cement, drawing calcium hydroxide to the surface, which then converts into calcium carbonate and turns pure white.
Calcium chemistry
Calcium atom (Ca) vs. Calcium ion (Ca2+)
The calcium atom, as found on the periodic table of elements, is relatively unstable due to its two valence electrons which are easily lost. When the electrons are lost, the atom becomes a calcium cation (Ca2+) because there are now two more protons than electrons.2 This stabilizes calcium and attracts it to things like carbonate (CO32-) so it can become neutrally charged. This process is called carbonation, and it creates calcium carbonate (CaCO3). The vast majority of calcium found in the world is in the form of calcium carbonate.3
Ca2+ + CO32- → CaCO3
Calcium ion + Carbonate ion → Calcium carbonate
But calcium can also attract other ions to stabilize itself. These include silica, phosphate and sulfate. As a result, when these substances oversaturate the water, different forms of scale begin to present themselves. But until they oversaturate the water, they stay soluble in water. And different calcium compounds have varying degrees of solubility. Calcium sulfate, for instance, is less soluble than calcium carbonate.
Calcium carbonate saturation and the LSI
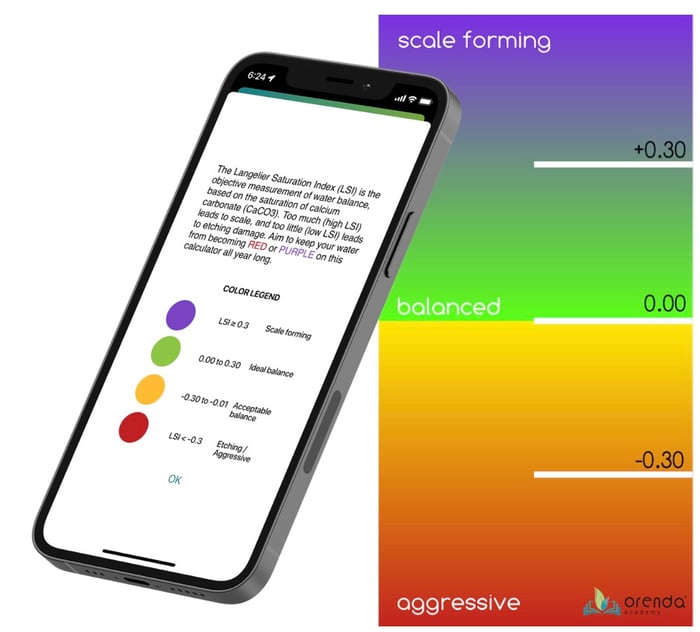
Calcium carbonate scale is the result of the oversaturation of CaCO3 in the water. The saturation of CaCO3 is measured using the Langelier Saturation Index (LSI). 0.00 is perfect equilibrium; below -0.30 is aggressive/undersaturated water, and above +0.30 is oversaturated/scale-forming water. So whenever there is calcium carbonate scale, it was caused by an LSI over +0.30 in one way or another.
Evaporation lines on tile are a common type of scale. Even though it's above the water line, technically it is a form of scale because water evaporated off the tile, leaving the calcium behind. As the water volume was reduced, the saturation of calcium increased (the LSI climbed) and the calcium carbonate precipitated on the tile (along with surface oils and nonliving organics).

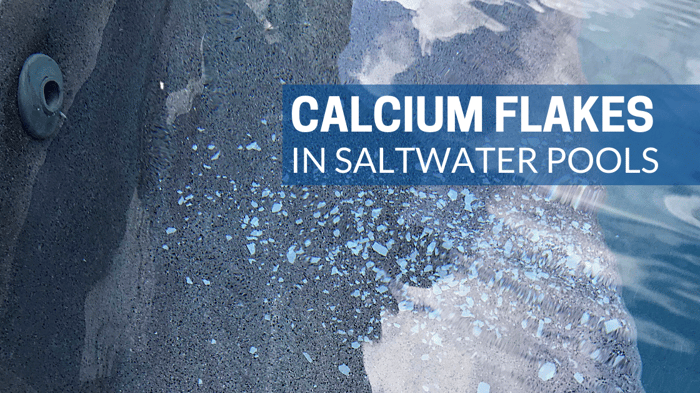
Calcium flakes in saltwater pools are another common form of scale. They are formed due to a localized LSI violation within the cell. Learn more about calcium flakes and how to prevent them here.
Contrary to popular belief, calcium hardness is usually not the driving factor leading to scale, unless the numbers are insanely high. Even with exceptionally hard water out of the tap, it still takes a combination of other LSI factors to drive calcium carbonate out of solution, like high pH, water temperature and/or carbonate alkalinity.
How to remove and prevent scale
Swimming pool scale removal
You can go about removing scale in at least two different ways.
First, you can physically try to remove it: grinding or bead-blasting can work on tile lines. You could also drop the pH sharply (so the water is more acidic), hoping to loosen the scale so it’s easier to break apart (though this has other consequences). More aggressive yet is to just use acid directly on the scale, which we do not recommend.
The problem with these strategies is that they don’t address the root of the problem–which is an LSI violation–so the scale usually comes back again. The other problem with these methods is the collateral damage done to your pool surface and equipment.
Option two—the better option—is to balance the LSI and use SC-1000 to chelate calcium in your water. Chelating calcium inhibits scale formation, but LSI balance is still needed to prevent scale entirely. If you already have scale, SC-1000 will gently break it down and pull it back into solution over time.
SC-1000 also flows through your system with the water, so it can remove scale in those hard-to-reach places—like your heat exchanger, salt chlorine generator, or the inside of your pipes. Lowering the pH of the pool to get slightly negative on the LSI (-0.29 to -0.01 LSI) can accelerate this process too. Do NOT go below -0.30 LSI, or else your water will become aggressive and can cause permanent damage.
.png?width=760&height=428&name=SC-1000%20(1).png)
So cleaning and treatment are both reactive measures. How about proactive?
Swimming pool scale prevention
As you may know, Orenda's message is about proactive pool care. We have four pillars of proactive pool care, and the first pillar is LSI Balance and Calcium Management. The article talks about how water craves equilibrium based on how saturated it is with calcium carbonate. With truly balanced water–as measured on the LSI–scale should not occur. If it does, there's something else going on...perhaps a faulty test kit reading.
In any case, it helps to understand water's relationship with calcium, because calcium is the most misunderstood chemistry in the pool business.
Be proactive and use the free Orenda App to control your pool chemistry. Doing so can prevent scale from ever starting, even with higher levels of calcium hardness.
Other types of scale
While calcium carbonate is the most common form of scale, there are a handful of other calcium compounds that can precipitate scale in and around swimming pools. They are relatively rare, thankfully. These compounds are much more difficult to remove, and they are not determined by the LSI, which only indexes calcium carbonate saturation.
Calcium Phosphate Scale

Another type of scale found in some commercial swimming pools is calcium phosphate scale (Ca5(PO4)3). It is quite rare, though it is often mistakenly identified, particularly in salt cells. For some reason, there is a widespread myth that calcium flakes in saltwater pools are calcium phosphate. But calcium flakes are calcium carbonate, and so far we have yet to see a single pool that had calcium phosphate in its salt chlorine generator.
And it's a good thing that calcium phosphate scale is rare, by the way. Calcium phosphate is much harder and more difficult to remove than calcium carbonate scale. It's an important ingredient in animal bones. Human tooth enamel, for instance, is hydroxyapatite, a form of calcium phosphate.4 On the Moh's 1-10 scale of mineral hardness, calcium phosphate is a 5, whereas calcium carbonate is just a 3. If you have calcium phosphate scale, it can be extremely difficult to remove and sometimes justifies replacing equipment entirely.
The most common places we have found calcium phosphate scale are in commercial swimming pool heaters and filters. Specifically on pools are maintained over 85ºF that use cal hypo as their primary type of chlorine. That, combined with high enough phosphate levels can lead to calcium phosphate scale.
Thanks to renowned water chemist Richard Falk, here is the formula for how much phosphate you need in your water in order to precipitate calcium phosphate scale:
PO4 = 10^[11.755 - log(CaH) - 2log(t) - (0.65 * pH)]
The temperature "t" is in Celsius.
So for 375 ppm CH, 7.5 pH and 80ºF (26.67ºC) we have
10^[11.755 - log(375) - 2log(26.67) - (0.65 * 7.5)] = 28.44 ppm = 28,440 ppb phosphate
The photo above shows two boulders on buckets. Those are large chunks of a sand filter that was hardened with calcium phosphate scale, and had to be fractured by an actual jackhammer to remove from the filter. The filter had to be cut open and replaced. Think about that.
Calcium Silica Scale


Calcium silica scale is sometimes formed on cementitious surfaces and is apparently immune to muriatic acid. At this time, we do not know much about silica scale, except that it exists and there is nothing we know of to chemically remove it. Silica scale often looks rounded, almost like kernels of rice, and can vary in color. As we discover more about this phenomenon, we will publish it and update this article.
Calcium Sulfate Scale Crystals


 Calcium sulfate scale looks like sharp crystals
Calcium sulfate scale looks like sharp crystals
Not to be confused with calcite crystals, which grow from the surface due to a very low LSI, calcium sulfate crystals are actually a scale deposit that is sharp and crystalline. And calcium sulfate is not directly related to the LSI. The LSI is the saturation of calcium carbonate, not calcium sulfate.
Calcium sulfate scale crystals are almost transparent at first, so they are very hard to see. They are also very sharp and can cut skin and bathing suits. While calcium sulfate is not directly correlated to the LSI, there is little doubt that they deposit because of an over-saturation of sulfate and calcium.5 We assume there is some sort of catalyst causing the formation to harden into crystals, but at this time we don't know what that is. We have been doing research on this, but none of what we have found was done with regard to swimming pools.6
Years ago, calcium sulfate was investigated by onBalance and was determined to be immune to muriatic acid, unless the acid is boiling. Thanks to the help of a retired chemist homeowner who contacted us through the Orenda help center, we now know that calcium sulfate can be softened with a high pH, not a low pH. And that's strange because it's the opposite of calcium carbonate scale, which acid can easily dissolve.
In December 2022, Orenda and Que Hales from onBalance experimented with sodium hydroxide to see if it would soften and remove calcium sulfate scale. It did soften the calcium sulfate crystals into a gel, making it somewhat easier to remove from filter grids. It did not completely remove it, however.
The most practical way to remove calcium sulfate scale seems to be physically sanding/grinding it off the surface. We wish we had better news for you. The best course of action is to prevent it by limiting the amount of sulfates you allow into your water in the first place7, and chelating calcium.
Conclusion
Scale is caused by an oversaturation of a given calcium compound in water. The most common calcium compound (by far) is calcium carbonate (CaCO3). The water's saturation of calcium carbonate is measured using the Langelier Saturation Index (LSI), so it's easy to prevent calcium carbonate scale by simply balancing the water according to the LSI. The Orenda Calculator™ was designed for this very purpose.
If the calcification you see is only on cement and nothing else around it, it is not scale of any type. Scale always is the result of an oversaturation, meaning the calcium compound comes out of the water and lands on surfaces.
You can inhibit scale formation by chelating calcium ions with SC-1000, which will help prevent calcium from binding to things ions like carbonate, sulfate, phosphate, or silica. If you have further questions, ask us in the comments below.
1 CK-12 Textbook. (2022). 6.10: Alkaline Earth Metals. Introductory Chemistry (CK-12). UC Davis LibreTexts.
2 Because elemental calcium (Ca) is considered unstable, it is usually more present as Ca2+. This is called a cation because it is positively charged, and therefore it seeks negatively charged ions like carbonate (CO32-) to bind to. The result is calcium carbonate (CaCO3), the most abundant form of calcium in the world. But to reduce confusion, we will call it calcium ion instead of calcium cation.
3 Calcium carbonate (CaCO3) is commonly called limestone and/or calcite. In swimming pools, it is also the most common form of scale (carbonate scale), as well as other calcification issues that are not scale (calcite crystals, uneven carbonation, etc.). Just because there is calcification in the swimming pool does not mean it's scale. Scale MUST precipitate from the water and land on surfaces, whereas calcium carbonate can also form as a result of aggressive water pulling calcium hydroxide to the surface, where it then gets carbonated into calcium carbonate (i.e. uneven carbonation, mottling/discoloration, or calcite crystals).
4 ScienceDirect. (2011). Various journal articles about tooth enamel.
5 Schierholtz, O.J. (1958). The Crystallization of Calcium Sulphate Dihydrate. Canadian Journal of Chemistry.
6 Here are some further resources for you to look into about calcium sulfate and how it forms crystals. If you can help us better understand how this information could pertain to swimming pools, please contact us.
7 According to the Pool Water Treatment Advisory Group (PWTAG) in the United Kingdom, sulfate levels over 300 mg/L (ppm) are problematic. Read their technical bulletin #3.
- Ravenhill, E., Kirkman, P., Unwin, P. (2016). Microscopic Studies of Calcium Sulfate Crystallization and Transformation at Aqueous-Organic Surfaces. Crystal Growth & Design. Ch. 16 (10), pp. 5887-5895.
- Lebedev, A.L., Kosorukov, V.L. (2017). Gypsum Solubility in Water at 25ºC. Geochemistry International. Volume 55, pp. 205-210.