Calcium Flakes in Saltwater Pools

If you own or maintain a saltwater pool, there's a good chance you have seen calcium flakes in it too. This article will explain what the flakes really are and debunk some myths about them. Let's get into it.
Covered in this article:
- How salt chlorine generators work
- Electrolysis
- Salt chlorine generators and the LSI
- Stagnant water
- Reversing polarity
- What are the white flakes in my pool?
- How to get rid of flakes in a saltwater pool
- Are calcium flakes dangerous in a pool?
- How to prevent flakes in a saltwater pool
- LSI Balance
- Contain pH with reduced alkalinity
- Flush out the salt cell with circulation
- SC-1000
- Conclusion
How salt chlorine generators work

We have a more detailed article about salt systems here, and we also elaborate on their chemistry and functionality in this article. The process of a salt chlorine generator––and its byproducts––explains why calcium flakes occur in so many saltwater pools.
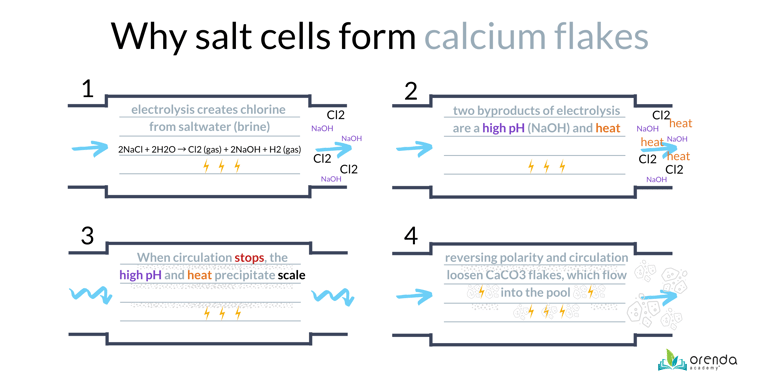
Saltwater pools use salt chlorine generators (also called Saltwater Generators or SWGs) that produce chlorine using electricity. Yes, saltwater pools are chlorine pools. They generate chlorine on-site. The process of using electricity to generate chlorine from saltwater is called electrolysis.
Electrolysis
Electrolysis has been around for over 100 years. Saltwater can conduct electricity better than fresh water. Salt cells have ruthenium-coated titanium plates (also called "fins" or "blades" that water passes between. One side is positively charged (cathode) and the other is negatively charged (anode).1 See the diagram below:

The electrolysis reaction in a salt chlorine generator looks like this:2
2NaCl + 2H2O → Cl2⇡ + 2NaOH + H2⇡
salt + water → with electricity⚡️yields→ chlorine (gas) + sodium hydroxide + hydrogen (gas)
This reaction, however, may be misleading because it does not account for how chlorine gas dissolves into the water, becoming two forms of acid:
Cl2⇡ + H2O → HOCl + H+ + Cl-
Chlorine (gas) in water yields Hypochlorous Acid + Hydrogen and Chloride
We could expand this even further and show how hydroxide and Chloride react, but it's just getting more complicated than it needs to be. What you need to know is that chlorine gas is created by electrolysis through saltwater (brine) and dissolves in water. Dissolved chlorine is acidic.
Related: Conductivity and Saltwater Pools
As you can see from the first reaction above, electricity in saltwater creates three new products: chlorine gas (Cl2⇡), sodium hydroxide (NaOH), and Hydrogen gas (H2⇡).
Chlorine is created at the anode, whereas the byproducts of sodium hydroxide and hydrogen are created at the cathode. And that brings us to our next point...
Salt chlorine generators and the LSI
Studying and applying the Langelier Saturation Index (LSI) has taught us that LSI violations are localized events. For example, carbonate scale always forms first in the highest-LSI places.
We often ask our customers, "Where does scale form first?" Almost everyone says something like "the tile line," "in the spa," or "on the spillway." But the truth is, in saltwater pools, the first place to form scale is usually in the salt cell or the heater. A salt cell, in particular, creates a high LSI because of how it works.
Most saltwater pools that have white flakes also have a high LSI. Most of those have a high LSI because they have too much alkalinity in their water. We'll come back to this idea later in this article.
Saltwater pools also have to be maintained differently than non-salt pools because the increased salinity adds at least 3000 ppm of TDS, which lowers the LSI in a noticeable way. Use the Orenda Calculator® and you'll see it for yourself.
Stagnant water
Calcium flakes are most likely to form immediately after the water stops moving. Think about it: there is high-pH sodium hydroxide still in the chamber, and the water just stopped moving. On top of that, the salt cell gets hot when it produces chlorine, so the temperature is increased as well.
And yes, calcification can occur when water is moving too, it just takes longer to build up.
Reversing Polarity
The high pH of sodium hydroxide creates a scale-forming condition on the cathode side of each blade within the salt cell. The chlorine gas dissolves quickly into an acid on the anode side.
Related 'From the Experts' Article: How to Avoid Salt Pool Maintenance Issues
This is important because salt cells reverse polarity. Reversing polarity means the positive electrode becomes the negative (the cathode becomes the anode) and vice versa. In other words, the electricity switches directions.
This occurs many times a day, and it loosens and fractures the carbonate scale that has adhered to the metal blades. The water flowing through the cell then carries those flakes of carbonate scale into the pool. This is why you see the flakes on the floor or benches near return inlets. In pools with attached spas, flakes are often found in the spa more than in the pool because of how the plumbing is designed.
What are the white flakes in my pool?

Every time we test calcium flakes, they are calcium carbonate (CaCO3). And with our knowledge of the LSI, calcium carbonate makes the most sense.
Because we already have so much information about calcium carbonate, we won't exhaust you here. Just know that it is by far the most common form of scale, and it is what every white flake in saltwater pools is made of...at least in our experience.
It should be noted that flakes are not a symptom of a flawed salt system or unique to a type of pool surface. A saltwater fiberglass pool can have flakes just as easily as a plastered or vinyl liner pool.
Do phosphates affect salt cells? Are the white flakes made of calcium phosphate?
However, there are several sources online that talk about these flakes like they are made of calcium phosphate (Ca3[PO4]2). So we looked into it.3 We have so far concluded that while calcium phosphate might be possible in salt systems, thankfully, it is rarely the case. Calcium phosphate scale is extremely hard to remove. The salt cell would need to be replaced.

We have seen calcium phosphate in commercial filters and heaters before—it's like concrete. Because of calcium phosphate formation in the filters, pools have literally had to cut open their filters and use jackhammers to get the sand out. See the photo of the sand boulders that were removed from a commercial sand filter only after being broken apart by a jackhammer.
If you really had calcium phosphate scale on your salt cell, it would be time to buy a new salt cell. That is unless you like using a hammer and chisel.
We have yet to see one saltwater pool with calcium phosphate in its cell or flaking into the pool. Ever. And we get calls every week about calcium flakes in saltwater pools. Sure, maybe that's 'anecdata' instead of actual data, but it's the truth.
We looked deeper into where this myth about calcium phosphate came from. We found several sources (which shall remain anonymous) that make the case that calcium flakes are calcium phosphate, and therefore, you should buy a phosphate remover.4
As you know, we are a manufacturer of a phosphate remover. While phosphates may seem to reduce the performance of salt chlorine generators, it's not a direct relationship. Nor are phosphates a driving factor for salt cell scale formation and flaking. Sure, it's possible to have calcium phosphate if you have levels high enough, but it's rare. Calcium phosphate is more likely to be found in a heater because of added contact time with heat.
There are plenty of reasons to remove phosphates, but scale in a salt system is not high on that list. But we agree it cannot hurt, and it can help prevent calcium phosphate from forming. Thanks to Richard Falk, the chemist we cite in many of our articles, below is a formula that shows how to calculate the level of phosphate needed for calcium phosphate to precipitate.5
PO4 = 10[11.755 - log(CaH) - 2log(t) - (0.65 * pH)]
In his example, the pool has 375 ppm calcium hardness, 7.5 pH, and 80ºF (26.67ºC). In that scenario:
10[11.755 - log(375) - 2log(26.67) - (0.65 * 7.5)] = 28.44 ppm = 28,440 ppb phosphate
28,440 ppb phosphates is really high and unlikely, but it is possible. We have seen a pool with over 40 ppm (40,000 ppb) before...once. Higher levels of calcium hardness, a higher pH and a higher temperature can all reduce the needed level of phosphate. So on paper, yes it is possible for calcium phosphate to form on salt cells...you just need really high levels.
Expanding on the relationship of phosphates and saltwater pools, salt and phosphates do not bind together. This is another myth that came from who-knows-where. Indeed, high phosphates do contribute to added chlorine demand, which is perhaps what people notice...but the phosphates themselves do not have anything to do with salt. Look at the formula above, and see if you can find the sodium or chloride in it. They are not there.
How to get rid of flakes in a salt pool

After the white flakes are blown into the pool through the returns, they can get dirty, tumble around, and begin to look more translucent. They are still calcium carbonate. When you vacuum them up, they may not look as crisp as when they first show up in the pool, but we believe they are the same flakes after being in the pool for several days.
Getting rid of the flakes can be done in a number of different ways.
You can chemically dissolve them with the right pool chemistry, but this takes time and might not be entirely practical. SC-1000 can help, but in the short term, you might be better off vacuuming the flakes and removing them from the pool.
Of course, we prefer to address the source of the flakes, too, which means you will need to clean the salt cell following the salt system manufacturer's instructions. These cleaning practices do not prevent future flakes, but they are a necessary step to complement the prevention strategy we'll explain in a moment.
Are calcium flakes in a pool dangerous?
We do get this question sometimes, and it can mean two different things. The answer to both questions is no. Calcium flakes are not dangerous in a pool.
- Calcium carbonate flakes that form in saltwater pools are not dangerous; they are just a symptom of an LSI violation within the salt cell.
- Calcium chloride flakes (77-80%) are frequently used to increase calcium hardness levels in water. They do get hot when dissolving, but apart from the heat, they are not dangerous and are used regularly.
Now let's talk about how to prevent calcium flakes.
How to prevent white flakes in a salt pool

There are proactive strategies to prevent calcium flakes from forming in the first place. We just need to prevent scale from forming in the salt cell. In order of importance, the strategies are LSI balance, containing pH, flushing the system out, and, if needed, using SC-1000. While this can help prevent scale, this may not prevent all salt system maintenance issues. But it should help you reduce the frequency of cleaning.
1. LSI Balance
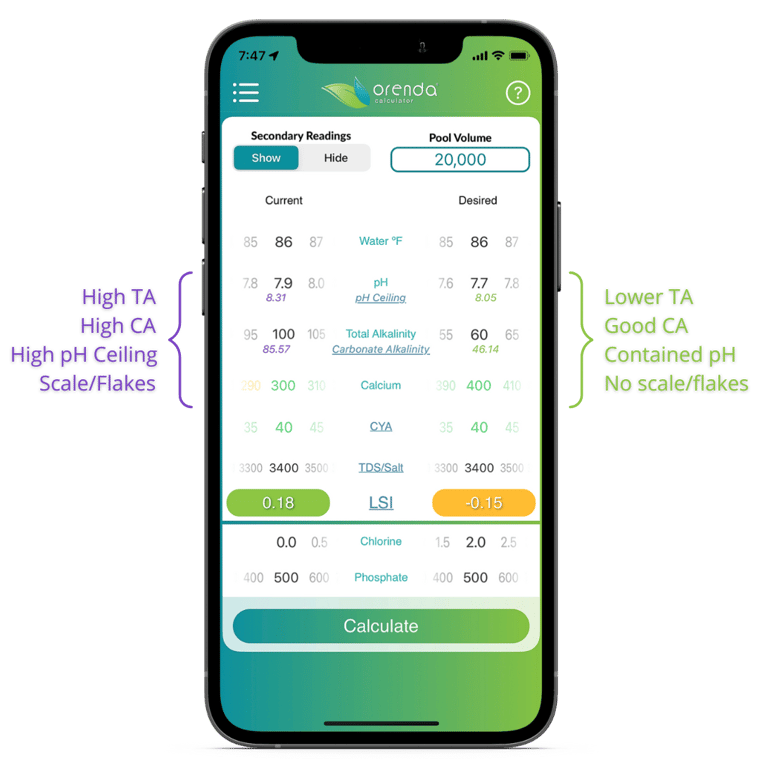
LSI balance is critical here. This strategy is probably the most important way to prevent scale flakes, and there are several ways you can do it using the Orenda Calculator®. We recommend a strategy of lower alkalinity and higher calcium to allow you to have a higher pH without forming scale.
2. Contain pH with reduced alkalinity
Instead of lowering your pH to 7.4 every week, consider lowering it to 7.6 or 7.7...maybe even 7.8. Thanks to physics, we know the pH will rise up to its equilibrium point (which we call the pH ceiling). Use this to your advantage!
Our recommended strategy for a saltwater pool is to manage the carbonate, (or crrected) alkalinity. If you have enough calcium hardness in your pool, the Total Alkalinity (TA) can be below 80 ppm in most cases. We recommend being in the lowest 10 ppm of the ANSI-11 standard for TA, which is 60-70 ppm. Salt pools with 60-something TA tend not to have many flakes (if any). On the Orenda Calculator®, it may look something like this:
Related: CO2, pH and Henry's Law
3. Flush out the salt cell with circulation
Stagnant water can be part of the issue with flakes, because when the pump shuts off, the salt cell may still be warm from producing chlorine, and the high pH created locally within the cell (sodium hydroxide) may still be sitting in there.
We recommend either switching to a variable speed pump to run continuous circulation, or program the system to shut the salt cell off a few minutes before the pump stops circulating. And depending on the salt cell manufacturer, most of them have some sort of a pressure/flow switch. When water is slowed down below about 25 gallons-per-minute, that flow switch should disengage, turning the salt cell off, while allowing slow water to continue flowing through it.
Any of these strategies will allow the cell to be flushed out and cooled down. An abrupt halt to water flow can be a major contributor to calcium buildup in the salt cell.
Another strategy to help with this is to reduce the cell's output percentage. We often hear of residential salt systems being run wide open (100% output) all the time. This maximizes chlorine production, but it also limits the time for the cell to be flushed out with fresh water. Reducing the salt system output to 40-80% is a good way to reduce the likelihood of calcium flakes.
4. Use SC-1000

If LSI balance and flushing out the cell are not enough, SC-1000 can help. Many pools are treated with SC-1000 weekly simply as a preventative maintenance dose to help keep calcium in solution, especially salt pools. Here's a video showing what it can do to an already-existing scale line:
If you have scale issues, here's our procedure for how to soften it and remove it chemically.
Additionally, if you're relying on your salt chlorine generator to handle all the non-living organics in the pool, there's a good chance it will eventually fall short. You can reduce the oils and other non-living organics that can coagulate in a salt cell and contribute to the problem.
Conclusion
The white flakes in salt pools are almost always calcium carbonate. These white calcium flakes look like crushed egg shells that blow into the pool through the return inlets. They occur because of a localized LSI violation within the salt cell, usually driven by too much alkalinity in the water.
When the salt cell reverses polarity, the carbonate buildup on the blades fractures and loosens, and flakes are carried by the circulating water out into the pool. Voilà, calcium flakes.
Cleaning flakes out can be time consuming and involves using diluted muriatic acid. Preventing flakes is actually easier. Prevention requires an LSI strategy to not only balance the pool today, but for the entire week until the next service visit.
Then, find a way to cool the salt cell down with added circulation time after the salt system shuts down. Think of this like a cool-down cycle. Finally, if you want extra help preventing scale, you can use SC-1000 on a weekly maintenance dose to prevent scale formation. We hope this article helps bring clarity to this topic. If you have more questions, feel free to contact us.
1 Titanium is used because it is less susceptible to corrosion. If the salt cell had iron or steel fins in them, they would not last very long. The ruthenium coating is used to maximize the production of chlorine gas from chloride ions. The activation energy required to produce chlorine is lower when using ruthenium as opposed to steel or other metals. Using a metal like iron, for example, would require much higher salt levels in order to produce chlorine.
2 Lowry, Robert W. 2009. IPSSA Intermediate Training Manual. Pg. 39
3 Lei, Y., Song, B., van der Weijden, R. D., Saakes, M., & Buisman, C. J. N. (2017). Electrochemical Induced Calcium Phosphate Precipitation: Importance of Local pH. Environmental science & technology, 51(19), 11156–11164. https://doi.org/10.1021/acs.est.7b03909
4 This evidently came about because people dissolved samples of these calcium flakes, then tested that water sample for phosphates. Indeed, phosphates were present. It's not yet clear to us how the phosphates got in there, but then again, we did not take the samples or do the experiment ourselves. All we're saying is we have yet to find actual calcium phosphate flakes in a pool, or even still stuck to a salt system. Every single case of flakes we have seen has been calcium carbonate. If that changes, we will update this article and talk about it in a future one.
5 Lenntech Technical Manual. FILMTEC Membranes Water Chemistry and Pre-treatment: Calcium Phosphate Scale Prevention. Pg. 2.
