Understanding ORP: Oxidation-Reduction Potential

Today, we're diving deep into the fascinating world of Oxidation-Reduction Potential (ORP). Whether you're a swimmer, a pool owner, or just someone with a thirst for knowledge, understanding ORP can shed light on the science behind maintaining clean and safe swimming pools.
Covered in this article:
- What is ORP?
- Oxidation and Reduction
- Why is ORP used in swimming pools?
- Why is ORP important?
- How is ORP measured?
- Elaborating on ORP probes and their functionality
- Factors that affect ORP
- pH and ORP
- Cyanuric Acid (CYA) and ORP
- Water Temperature on ORP
- How to increase ORP in swimming pools
- Conclusion
What is ORP?

Oxidation Reduction Potential, or ORP, measures a substance's ability to oxidize or reduce another substance in a solution. In simpler terms, ORP is a way to quantify a solution's electron transfer capability.
Chlorine is the most common residual disinfectant and oxidizer used in swimming pools. When chlorine dissolves in water, it dissociates into the strong, active killing form of chlorine, Hypochlorous acid (HOCl), and its much weaker counterpart, Hypochlorite ion (OCl-). Both are oxidizing and killing agents, keeping the water safe, clean, and clear.
ORP is technically a thermodynamic measurement, not a kinetic one. But for simplicity, it's helpful to describe ORP with a visual analogy.
If chlorine is a car, ORP is not the speed of the car, it's more like the quality of the highway. It tells us how well the car can potentially drive. Low ORP is like a bumpy gravel road with sharp turns. The higher the ORP, the more smooth and wide-open the highway becomes, so the car can drive faster. Higher ORP means chlorine can kill and oxidize faster and more effectively.
Oxidation and Reduction
For every action, there is an equal and opposite reaction. Oxidation and reduction are complementary reactions that occur simultaneously. Together, they are called a Redox reaction. One substance loses electrons as it's oxidized, while the other gains electrons and is reduced. This complementary relationship ensures the overall neutrality in the solution as electrons move around.
When a substance is oxidized, it loses electrons. When a substance is reduced, it gains electrons. These redox reactions can be remembered by the acronym OILRIG: Oxidation is Loss, Reduction is Gain.1
It sounds backward (we know). How does a substance get "reduced" when it "gains" something? The answer is that electrons (e-) have a negative charge, and they reduce the oxidizer's charge. So when we say "chlorine gets reduced", we are referring to it getting used up oxidizing or killing contaminants in the water.
Why is ORP used in swimming pools?
ORP is used in swimming pools to gauge disinfection and oxidation performance in real-time. Most commercial pools use pH and ORP probes 24/7 because it's important for pool operators to know if something is wrong. If ORP drops, it's a signal that chlorine might be falling behind or that the oxidant demand in the water has increased relative to the amount of free chlorine (and secondary oxidizers like Ozone or AOP, if used) in the water.
Why is ORP important?
ORP is a valuable tool for pool operators trying to maintain the safety and cleanliness of water. By monitoring ORP levels regularly, we can ensure the water is properly disinfected, which minimizes the risk of waterborne illnesses. It also gives operators a sense of the chlorine demand in the water. A high ORP indicates chlorine is on top of things, and a low ORP lets an operator know chlorine is struggling to keep up.
How is ORP measured?
ORP is measured using a specialized device called an ORP probe (or ORP meter). This probe has electrodes submerged in the solution being tested—–in our case, swimming pool water. The electrodes measure the voltage generated by the electron transfer reactions (called redox reactions) in the water. This voltage is converted into an ORP reading, usually expressed in millivolts (mV).
Many states list 650 mV as the minimum acceptable ORP level for a public swimming pool. Over 750 mV is very good, and over 800 mV is exceptionally good. The higher the ORP mV, the better chlorine performs and, therefore, the safer the water.
Elaborating on ORP probes and their functionality

ORP probes operate on the principle of electrochemistry, where electron transfer reactions occurring in the solution are measured to determine the solution's ORP.
An ORP probe consists of electrodes typically made of noble metals such as platinum or gold. These electrodes are immersed in the solution being tested. Electron transfer reactions occur at the electrodes' surface when the solution contains oxidizing or reducing agents.
For example, in a chlorinated pool, water molecules may undergo oxidation at the anode (positive electrode), losing electrons to form oxygen gas (O₂) or other oxidizing species.2 Chlorine molecules undergo reduction at the cathode (negative electrode), gaining electrons to form hypochlorite ions (OCl⁻).
The ORP probe measures the potential difference generated by these electron transfer reactions. This potential difference, expressed in millivolts (mV), is directly proportional to the solution's ORP. This is sometimes also called conductivity, though conductivity and ORP are not the same thing.3
Factors that affect ORP
pH and ORP
ORP is highly sensitive to pH, both with and without cyanuric acid in the water.4 Because pH is about the movement of Hydrogen ions (H+), it affects the electrical charge of different substances in water and, thus, the movement of electrons. The movement of electrons is what ORP is all about.
HOCl + H+ + 2(e-) ⇄ Cl- + H2O
Hypochlorous Acid + Hydrogen ion + 2 electrons ⇄ Chloride ion + water
Notice how the above reaction has both HOCl and the Hydrogen ion (H+) on the left side of the reaction. This means the ORP will drop when either HOCl or the Hydrogen ion is lower. So higher pH makes the ORP drop whether CYA is present or not, and whether HOCl is lower or not.
This is important to recognize because if a consistent pH is not maintained, the ORP reading is effectively useless. Its baseline is moving. This is why commercial pools set a desired pH and use an acid or CO2 feeder to try to suppress it and keep it steady, even though we know pH cannot be controlled.
For ORP to be helpful and relevant to a pool operator, it has to be correlated with a known free chlorine (or free chlorine to cyanuric acid ratio) at a fixed pH. Since pH cannot be controlled, a chemical controller must work 24/7 to suppress it and keep it as steady as possible.
Cyanuric Acid (CYA) and ORP
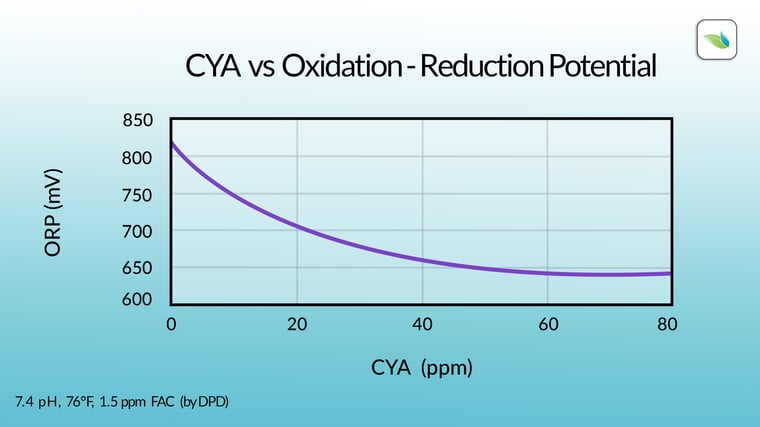
Cyanuric acid lowers ORP levels. The more CYA in the water, the lower the concentration of Hypochlorous Acid (HOCl), and therefore less active oxidizer is present in the water. It's that simple. And it doesn't take a lot of CYA to drastically reduce the concentration of HOCl in the water.
Look at the difference between the red line (%HOCl) on the two charts below:

We discuss more about chlorine, pH, and CYA relationships here.
Water Temperature and ORP
Since ORP is a thermodynamic measurement, water temperature matters. The colder the water, the slower chemical reactions like oxidation and reduction can occur, thus the ORP decreases. The warmer the water, things speed up, and the ORP increases.
Salt chlorine generators and ORP
Saltwater pools with ORP systems often experience a phenomenon known as "ORP suppression". When the salt system actively produces chlorine, the ORP can drop and stay low until the salt system shuts off. The ORP then slowly recovers.
The most plausible theory is that the Hydrogen gas (H2⇡) bubbles produced in the salt cell interfere with the ORP probe, depressing the ORP level. while this hasn't yet been proven definitively, it chemically makes sense to us.5
How to increase ORP in swimming pools
Pool operators can do a few things to optimize ORP levels in a swimming pool. To increase ORP, operators can:
- Increase free chlorine levels. The more free chlorine, the more HOCl present.
- Enhance Circulation: Improving water circulation and mixing can help distribute chlorine more evenly throughout the pool, increasing its effectiveness in sanitizing the water. Increasing turnover rates also means the water will be filtered and treated more regularly.
- Maintain lower pH: As discussed earlier, especially in indoor pools without cyanuric acid, pH directly impacts chlorine strength (%HOCl) and the ORP itself. While we believe pH levels can (and should) be slightly higher in outdoor, stabilized residential pools, commercial pools with chemical controllers and ORP systems should maintain
- Minimize Contaminants: Regularly removing organic contaminants such as leaves, debris, and body oils from the pool water can reduce the demand for chlorine and help maintain higher ORP levels.
By implementing these strategies and closely monitoring ORP levels, swimming pool operators can ensure that their pools remain clean, safe, and enjoyable for swimmers of all ages.
Conclusion
Public pools almost always have some form of chemical automation. This allows pool operators to keep water safe and disinfected 24/7. Most of these controllers will have both a pH and ORP probes, giving real-time readings of the current state of the water.
ORP does not replace the need to test free and total chlorine levels, but it gives a good picture of how burdened chlorine is in the water. The higher the ORP, in millivolts (mV), the more effective oxidation is. The lower the ORP, the more chlorine struggles to keep up with the demand. A low ORP is cause for concern if/when levels dip below 650 mV.
1 Oxidation and reduction reactions are often called Redox reactions (reduction, oxidation). The two happen in tandem. The oxidizer steals and gains electrons, so it gets reduced (because electrons (e-) have a negative charge). The oxidant loses electrons and sometimes gains Oxygen, getting oxidized. For chlorine oxidation or disinfection, the active form of HOCl will swap its oxygen for an electron, reducing HOCl into HCl, which can no longer oxidize.
2 Vitz, E., Moore, J., Shorb, J., Prat-Resina, X., Wendorff, T., Hahn, A. (publication date unknown). 17.2: Electrolysis. Chemical Education Digital Library (ChemEd DL). Adapted for LibreTexts online learning.
3 Conductivity is a specific measurement in water, but the term is used loosely in the aquatics industry. It is technically a measurement of water's ability to conduct an electrical current, which is primarily driven by the concentration of dissolved ions in water. Conductivity is used to approximate Total Dissolved Solids (TDS) and salinity because both impact how well electricity can travel through water. Conductivity alone cannot distinguish between salts and other dissolved ions, but it's a good approximation.
Conductivity and ORP are two different things. ORP measures the potential for redox reactions and is related to the presence of oxidizers like chlorine, ozone, and hydroxyl radicals in the water.
4 In non-stabilized pools (meaning no cyanuric acid), like indoor pools, the pH not only impacts ORP, but it directly impacts the concentration of Hypochlorous acid (HOCl) relative to Hypochlorite ion (OCl-). The higher the %HOCl, the better the chlorine performs, and the higher the ORP. This still happens in stabilized pools, but not as much. The %HOCl in a stabilized pool is hardly affected by pH at all, which we elaborate on in this article. That said, the pH also affects ORP directly, with or without CYA.
5 It should be noted that the salt levels in the water do not significantly affect ORP, even though they directly impact conductivity. This is a common misunderstanding in the pool industry. See our footnote (3) above, explaining how conductivity and ORP differ.
