How to Reduce Cyanuric Acid in a Swimming Pool

High levels of Cyanuric Acid (CYA) in pool water can cause issues with both water quality and water balance. Overstabilization slows chlorine down and lowers the LSI, making water more aggressive. Because of these reasons and more, keeping CYA to a minimum is our Fourth Pillar of Proactive Pool Care.
Covered in this article:
- How to reduce cyanuric acid in pools
- Is there an effective cyanuric acid reducer product?
- What is Cyanuric Acid (CYA)?
- CYA protects chlorine from sunlight, but it also slows chlorine down
- The Model Aquatic Health Code (MAHC) CYA limit for public pools
- CYA lowers the LSI
- How do pools get high CYA levels?
- Conclusion
How to reduce CYA in pools

CYA will eventually be oxidized from the water, but it takes months and high chlorine levels. In short, cyanuric acid will break down into an organic nitrogen compound similar to urea. Many more oxidation and substitution reactions occur, using up chlorine in the process. Eventually, CYA will be broken down enough to combine with chlorine as part of the breakpoint chlorination process.
The most effective CYA removal methods––as of now––are draining/diluting water, and reverse osmosis filtration (RO).
Is there an effective cyanuric acid reducer product?
Some CYA reducers are on the market, but our customers have told us their results are mixed. We hope the technology develops into something practical because CYA overstabilization is a big problem for millions of outdoor pools.
One such product is a type of nitrifying bacteria capable of breaking down cyanuric acid into its organic nitrogen components and then removing the nitrogen. We have not found enough information publicly available to tell exactly what it does once the CYA is broken down. We know that these nitrifying bacteria cannot survive in chlorinated water. So the pool must be dechlorinated before use, and the temperature must be warm enough for the bacteria to work. From what we are told, the results vary.
There is also some evidence that Aluminum sulfate (Al2(SO4)3) flocculation can help remove CYA from water. This also has mixed results but some of our customers have told us they have had some success using Alum.
So is there an effective cyanuric acid reducer on the market? As of now, not really. But we are optimistic that there will be one. Alum seems to be the best option outside of draining and reverse osmosis.
But what is CYA, and why is it important to know how to remove it from swimming pools?
What is Cyanuric Acid (CYA)?

Cyanuric acid (CYA, stabilizer, or conditioner) protects chlorine from sunlight degradation.1 Without sunlight protection, free chlorine will degrade significantly in a short amount of time. Chlorine's half-life is approximately 20-45 minutes in sunlight (depending on variables like water depth), which means the chlorine level will be cut in half every 20-45 minutes. Without CYA, all the chlorine will be gone in about three hours.
It does not matter if it's a bright, sunny day or a cloudy day. The UV rays that destroy chlorine penetrate clouds. Just as we can get sunburned on a cloudy day, sunlight can also destroy chlorine. Some sunlight protection is beneficial because of the critical importance of maintaining a residual sanitizer in the water. But too much of a good thing becomes a bad thing.
Below is a graphic we recreated from a Kent Williams white paper and overlayed the CDC's recommended 15 ppm CYA limit.3 The curve illustrates that low levels of CYA are beneficial for chlorine protection. However, as CYA levels continue to increase, those benefits diminish. There is hardly a difference between the sunlight protection 30 ppm CYA provides (~98%) and anything above it.
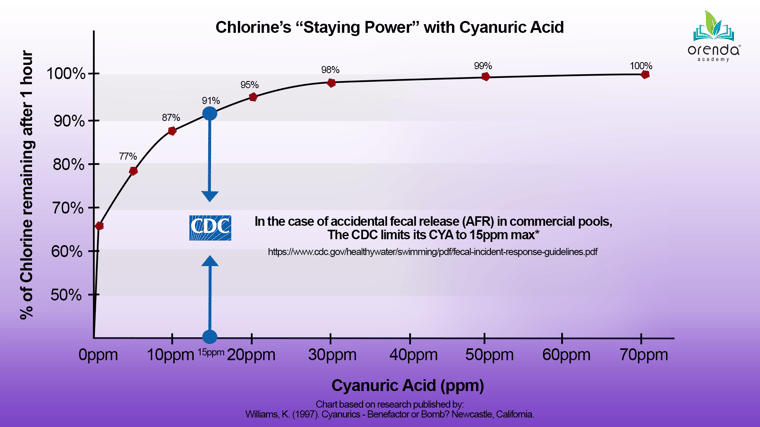
As you can see, just 10 ppm protects 87% of chlorine, 20 ppm protects 95%, and 30 ppm protects 98%. To explore this concept more deeply, check out our article about free chlorine, pH, and CYA relationships. The chart above shows us that a little CYA is good. Now, let's explore why too much CYA is bad.
CYA protects chlorine from sunlight, but it also slows chlorine down
There is abundant scientific research about cyanuric acid's impact on chlorine. We have cited several peer-reviewed articles throughout this article. The research is clear: stabilized chlorine is slower than non-stabilized chlorine.1,2,3,4,5,6,7,8,9 The more CYA in the water, the slower the chlorine. At some point, there is enough CYA that chlorine cannot keep up with demand, and that condition is called overstabilization.
As we just learned, chlorine will not last very long without some CYA in the water. So the key is to have minimal CYA in the water. That way, most of our chlorine is protected from UV degradation, and we also do not slow our chlorine more than necessary. It's a trade-off that outdoor pool owners and operators must decide how they want to handle.
There is a difference between using chlorine and losing chlorine.
We have another article about chlorine, CYA, and temperature relationships. Take a few minutes to read it because it addresses a common myth about chlorination. The belief is that warmer days (brighter sun, hotter weather, warmer water, etc.) burn out chlorine faster; therefore, more CYA is required to protect chlorine. And while that appears to be true, it is false. Coincidence is not the same as causality.
There is a difference between using chlorine and losing chlorine to sunlight. In warmer temperatures, chlorine demand increases, and chlorine performance and speed also increase. So chlorine is being used faster. Temperature alone has nothing to do with CYA's ability to protect chlorine from sunlight. So while chlorine levels do get depleted in warmer weather, it's often because chlorine is being used faster, not being lost to sunlight degradation.
Unfortunately, this belief leads pool professionals and owners to increase CYA levels to try and hold chlorine longer. In the case of pool pros, this means trying to hold chlorine for a week because they visit each pool once a week. The truth is, more CYA may help hold chlorine for a week, but it's by slowing chlorine down, not by offering it noticeably more sunlight protection.
Alkalinity is also related to chlorine loss to sunlight because the higher the carbonate alkalinity, the higher the pH ceiling. This means more chlorine will break away from CYA in the water and that sunlight can destroy chlorine. It can get complicated. So we explain it here in this Rule Your Pool podcast episode 128:
The Model Aquatic Health Code CYA limit for public pools
Because CYA significantly slows chlorine's kill times, the contact times (CT values) required to kill pathogens increase.2,5,6,7,9 This is where health departments and the CDC come into play. The primary purpose of chlorinating water is to disinfect it, preventing Recreational Water Illnesses (RWIs) like Cryptosporidium (crypto).2,5,6
The CDC's Council for the Model Aquatic Health Code has published recommendations to limit the cyanuric acid level in public pools to 15 ppm in case of a fecal accident (see the chart above).10 This is due to the increased contact times required to inactivate cryptosporidium.
In speaking with some members of the CMAHC ad-hoc committee on Cyanuric Acid, this recommendation was a practical limit because the contact times required to inactivate Crypto became too high to be useful. Operators with a diarrheal accident (aka "code brown") must drain and dilute their pool to get CYA levels low enough for a chlorine shock to be effective.
CYA lowers the LSI
Another consequence of CYA is its impact on water balance. The Langelier Saturation Index (LSI) formula calls for carbonate alkalinity (or corrected alkalinity). This means contributors to total alkalinity–namely cyanurate alkalinity–must be subtracted. The Orenda Calculator™ does all of this math for you automatically.
More CYA > more cyanurate alkalinity to subtract from total alkalinity > lower carbonate alkalinity (relative to total alkalinity) > lower LSI > more aggressive water.
Play around with the LSI calculator, and the LSI value decreases when you increase CYA. Be mindful of this.
How do pools get high CYA levels?
Cyanuric acid is not naturally occurring and should not be in tap water. All the CYA in a swimming pool was either added directly (granular or liquid CYA products), or as a byproduct of stabilized chlorine (sodium dichlor or trichlor).
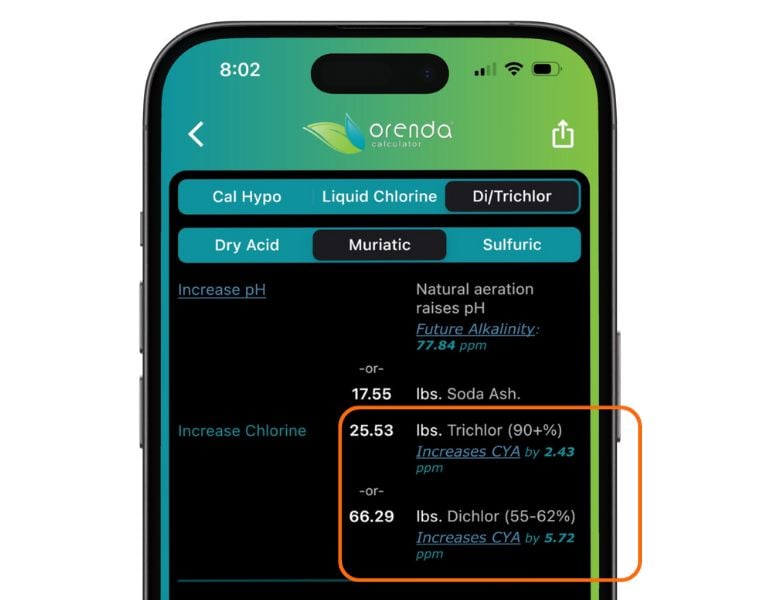
Overstabilization almost always happens because of trichlor and dichlor use, not granular or liquid CYA products. Those are measured amounts of CYA, and our calculator shows exactly how much is needed to achieve a desired CYA level. Trichlor and dichlor, however, continually raise CYA the more you use them.
For instance, trichlor is only 45.8% chlorine (by weight). The majority of it is cyanuric acid.

Conclusion
Minimal CYA is a good thing because it protects chlorine from sunlight degradation. But too much CYA is bad, slowing down chlorine and lowering the LSI.
To avoid overstabilization, minimize the use of stabilized chlorine (trichlor and dichlor). If CYA levels are high, the most economical way to reduce CYA is to drain and dilute. Reverse Osmosis filtration is also effective, but expensive.
It really just comes down to cost and risk. Significant draining has risks and should only be done by a pool professional, so dilution over time is often the best option.
1 County of Sacramento Health Department. (2014). Fact Sheet on Cyanuric Acid and Stabilized Chlorine Products. Environmental Management Department.
2 Falk, R.A., Blatchley, E.R., III, Kuechler, T.C., Meyer, E.M., Pickens, S.R., Suppes, L.M. (2019). Assessing the Impact of Cyanuric Acid on Bather’s Risk of Gastrointestinal Illness at Swimming Pools. Water. Volume 11, 1314.
3 Williams, K.M. (2000). Cyanurics - Benefactor or Bomb? Executive Director of the Professional Pool Operators of America.
4 Canelli E. (1974). Chemical, bacteriological, and toxicological properties of cyanuric acid and chlorinated isocyanurates as applied to swimming pool disinfection: a review. American journal of public health, 64(2), 155–162. https://doi.org/10.2105/ajph.64.2.155
5 Shields, J. M., Arrowood, M. J., Hill, V. R., & Beach, M. J. (2009). The effect of cyanuric acid on the disinfection rate of Cryptosporidium parvum in 20-ppm free chlorine. Journal of water and health, 7(1), 109–114. https://doi.org/10.2166/wh.2009.008
6 Murphy, J. L., Arrowood, M. J., Lu, X., Hlavsa, M. C., Beach, M. J., & Hill, V. R. (2015). Effect of cyanuric acid on the inactivation of Cryptosporidium parvum under hyperchlorination conditions. Environmental science & technology, 49(12), 7348–7355. https://doi.org/10.1021/acs.est.5b00962
7 Wojtowicz, J. (2001). Relative Bactericidal Effectiveness of Hypochlorous Acid and Chloroisocyanurates. Journal of the Swimming Pool and Spa Industry. Vol. 2(1), pp. 34-41.
8 Wojtowicz, J. (2004). Effect of Cyanuric Acid on Swimming Pool Maintenance. Journal of the Swimming Pool and Spa Industry. Vol. 5(1), pp. 15-19.
9 Anderson J. R. (1965). A study of the influence of cyanuric acid on the bactericidal effectiveness of chlorine. American journal of public health and the nation's health, 55(10), 1629–1637. https://doi.org/10.2105/ajph.55.10.1629
10 CDC (2019). 2023 Model Aquatic Health Code: Code Language. Council for the Model Aquatic Health Code (CMAHC). 4th Edition. Page 182, §6.5.3.2.1A
