Cold Water and the LSI

After the initial fill/startup, the vast majority of damage done to a swimming pool occurs during the winter. This is because cold water lowers the LSI, making water more aggressive. In other words, etching and corrosion are more likely to happen in cold water.
Covered in this article:
- Water temperature's impact on the LSI
- Winter consequences
- Calcite crystals
- Winter dust
- Ghosting
- Winter consequences
- Cold water chemistry, the Orenda way
- Winterizing pools the Orenda way
- Conclusion
Water temperature's impact on the LSI
The colder the water, the lower the Langelier Saturation Index (LSI). This means water is more aggressive and hungry for calcium. Aggressive water is more likely to etch cement to get the calcium it craves. The lower the temperature, the more aggressive the water.
Water temperature is the easiest of the six LSI factors to measure, yet it is the most neglected. Why? Because most pool test kits do not include a thermometer. Therefore, most people do not even think about water temperature. But we assure you, temperature matters! Unlike most substances, which dissolve better in hot water, calcium dissolves better in cold water.
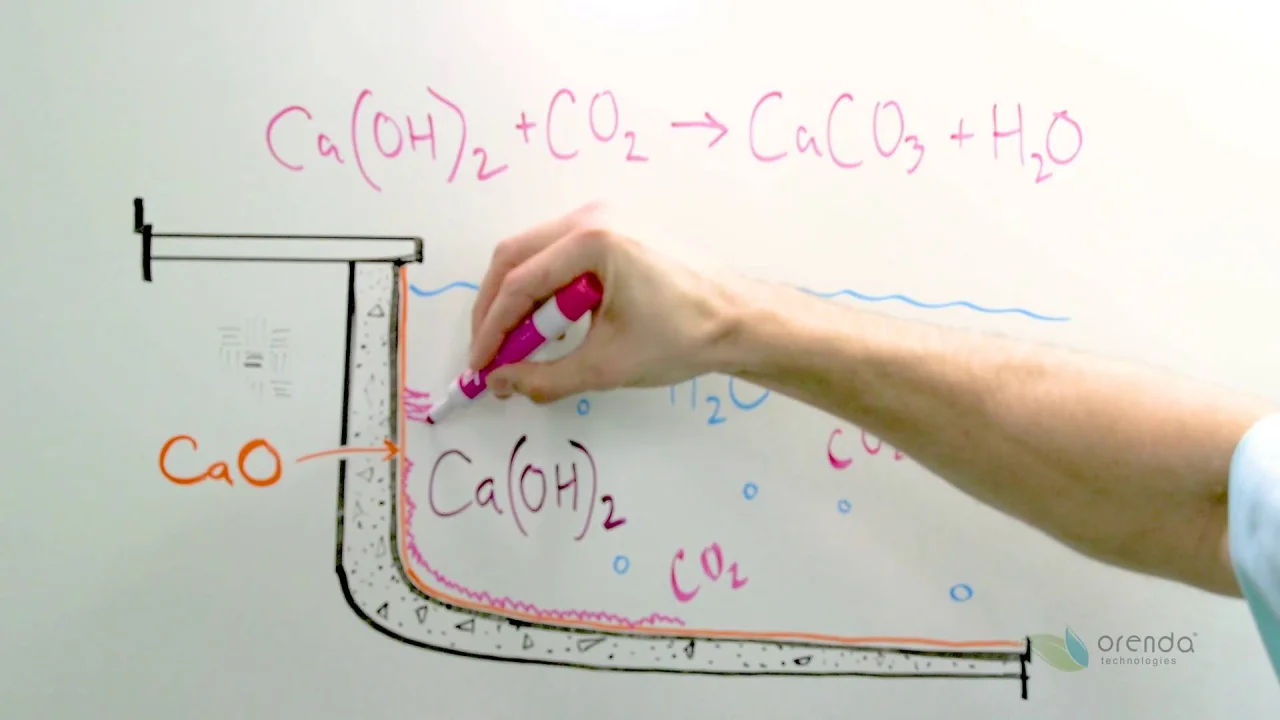
Winter consequences
Let's take a look at the Orenda Calculator™ to illustrate what cold water does to the LSI.
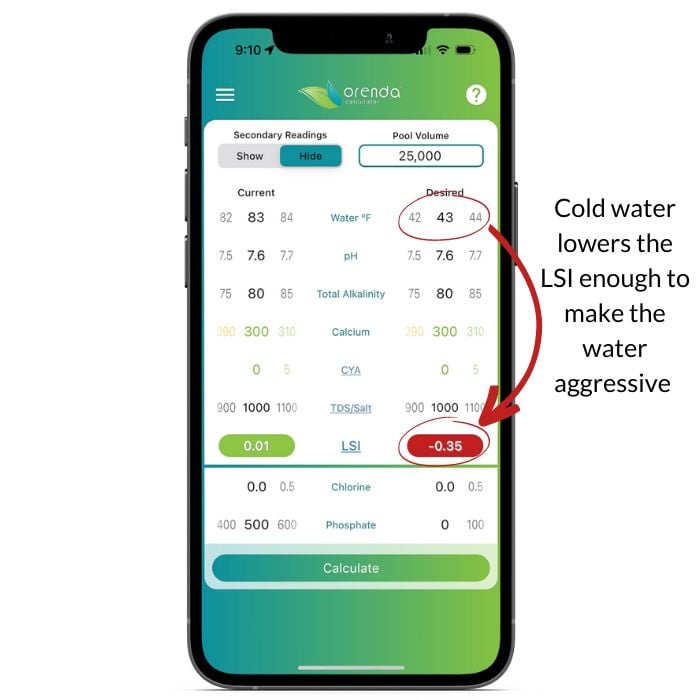
As you can see, simply dropping the temperature 40ºF made balanced water become unbalanced and aggressive. Plan accordingly, because if your LSI is red on the Orenda Calculator™, that's when damage occurs.
We commonly see three types of plaster damage resulting from cold water during the winter lowering the LSI too much. They are calcite crystals, winter dust, and what we call "ghosting". Sometimes all three can occur, or any combination of them. Note: these issues are just in pools with cementitious finishes (plaster, quartz, pebble, etc.). Vinyl liners can fade and wrinkle, and fiberglass pools can deteriorate and turn white, which we call "chalking".1
Calcite crystals

Calcite crystals are outgrowths of calcium carbonate (CaCO3) that are pulled from the cement by aggressive water. They are NOT scale. We have had several samples of crystals laboratory tested, and the lab has concluded these crystals grow out of cement, as opposed to being deposited on top of it.
Winter dust

Winter dust is usually found when reopening a winterized pool that had a solid cover on it, rather than mesh (or no cover). Our theory is the water gradually dissolves calcium as the temperature drops the LSI below -0.30. It continues to eat until the water reaches its coldest temperature, then the LSI remains balanced during the winter–at the expense of the cement in the pool finish. But as the water warms up in the spring, the LSI rises. Eventually the LSI rises high enough to begin precipitating calcium dust all over the floor of the pool.
Winter dust tends to go hand-in-hand with what we call "ghosting".
Ghosting

"Ghosting" is the term we think best describes this issue...but there is probably a better name for it. Depending on the cause, it can be uneven carbonation during plaster curing, or it can be caused by aggressive water during the winter. Either way, aggressive water is the culprit, and we know that because this carbonation occurs around aggregates like pebbles. Ghosting is when low-LSI water dissolves the cement, which pulls calcium hydroxide (Ca(OH)2) to the surface, which then gets carbonated into calcium carbonate (CaCO3). Calcium carbonate is pure white.
We know this is from aggressive water because if it were scale, the calcium carbonate would be on top of everything. Scale originates from the water due to an over-saturation (LSI above +0.30).
Look closely at ghosting and you will see white rings around the aggregates (i.e. pebbles), indicating the loss of calcium hydroxide from the interfacial transition zone (ITZ).

Cold water chemistry, the Orenda way
One of the most common questions we get is "what should calcium hardness be in my pool?" The answer always depends on your water temperature throughout the year. Colder places where pools will freeze need more calcium. For instance, pools in Michigan need more calcium than pools in Miami.
The ideal calcium hardness level is one that allows you to maintain LSI balance year-round. That means accounting for the coldest water temperature your pool can have. If you know your pool will freeze, that's great news! You know the worst-case scenario and you can plan accordingly. It's easy with the Orenda Calculator™. The calculator will not only show you what different calcium levels will do to your LSI, it will show you what all the factors can do. You have plenty of options to balance your water when it's cold.
If your water will not freeze, you still need to know the water temperature every time you treat the pool.
All we're saying is you have a choice to make. Either you balance the water, or the water will balance itself. While you have over 720 different combinations of six factors to choose from, water has only two ways to balance itself: eat or scale.
Our suggestion is to lean on calcium hardness because it's the bedrock of the LSI. The colder the water, the more you need. Do not be afraid to load the pool with 500+ ppm of calcium hardness if you know the pool is going to freeze. Feed the bear before it hibernates, because if you don't, it will wake up hungry in the middle of winter. In this analogy, the water in your pool is the bear (in case the analogy wasn't obvious).
Guiding chemistry ranges based on water temperature
We at Orenda prioritize LSI first, and range chemistry second. Ranges can be fine as long as they are in the context of the LSI. Keep that in mind. The time of year often determines a pool's water temperature, and that temperature determines what the other chemistry parameters need to be.
Pool Startups almost always involve cold water

Unless you're filling the pool with water from a truck that has been sitting in the sun for hours, most fill water is cold. It flows through pipes underground, and usually, groundwater is around 55º-65ºF. If you have no water thermometer, we recommend you buy one and use it before every fill.
We have written extensively on the importance of an LSI-focused pool startup to prevent pool plaster problems. In short, the LSI really matters, so you have to account for factors like temperature when deciding what to add to the water that initially fills a new or freshly resurfaced pool. It's critical to the integrity of the surface.
This also means recognizing the time of year. Is it October and the water is getting colder by the day? Or is it May and the water is getting warmer by the day? In any event, factor in the tap water temperature when calculating its LSI for the startup.
Winterizing pools the Orenda Way
Since we know cold temperatures make the water more aggressive, all we need to do is account for the temperature. Usually, this means increasing calcium hardness and allowing pH to naturally rise up to the pH ceiling. You might want a bit more total alkalinity too, but it all depends on where your chemistry is before the temperatures drop.

If you have a mesh cover (or no cover), rain and snow will eventually end up in the pool, and will dilute the chemistry. Pay attention to the type of cover you have, and follow our procedure to winterize the pool properly.
Related: How to Winterize a Pool the Orenda Way
Conclusion
Whether your pool freezes in winter or not, water temperature still matters. Cold water temperature lowers the LSI, which makes water more hungry for calcium. Either you provide it the calcium saturation it craves, or the water will have to find it on its own. The consequences, as outlined in this article, are permanent damage.
1 One day we will write about these conditions in separate articles. Hopefully, we will remember we referenced them here and will come back to edit this post to link to them. But we are notoriously bad at such things.
