Understanding Breakpoint Chlorination

Breakpoint chlorination is an important concept in pool chemistry. Let's explore what it means and the difference between breakpoint chlorination and superchlorination (shocking).
Covered in this article:
- What is breakpoint chlorination?
- Chlorine vs. nitrogen compounds
- Beyond the breakpoint
- Superchlorination
- Nitrogen compounds
- Conclusion
What is breakpoint chlorination?
Breakpoint chlorination is a threshold where free chlorine levels exceed the amount required to destroy nitrogen-based oxidants.1 Past this threshold, a residual of free available chlorine (FAC) can build. Theoretically, exceeding the “breakpoint” prevents increased levels of disinfectant byproducts––namely, combined chlorine, aka chloramines.
Chlorine vs. nitrogen compounds
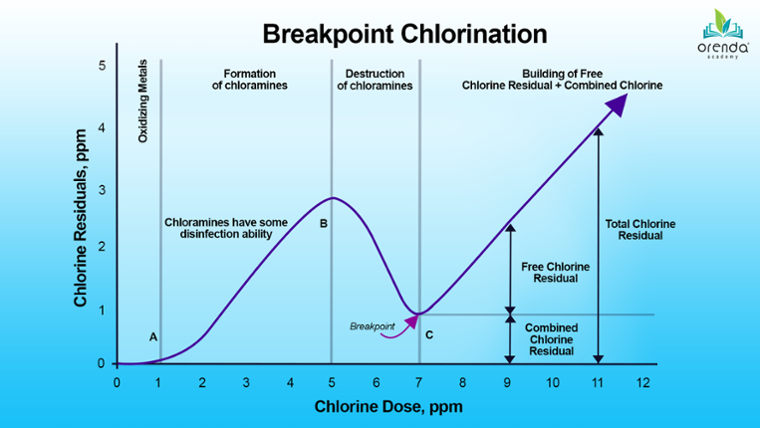
Look at the graph above. When chlorine is first added to water, it immediately begins to oxidize metals like iron and manganese, which reduces chlorine in what is called a redox reaction.
This initial reaction wipes out a certain portion of chlorine, so nothing shows up on the graph until point (A). As more chlorine is added to water, it oxidizes other contaminants—not just germs, but non-living organics and nitrogen compounds too. These oxidants create byproducts.
In particular, we're talking about nitrogen compounds because chlorine must combine with nitrogen compounds to destroy them. Hence, the term combined chlorine. Inorganic Ammonia (NH3) and more complex nitrogen compounds (like urea) combine with chlorine and create byproducts called chloramines.
Let's start with ammonia and examine how it combines with chlorine.
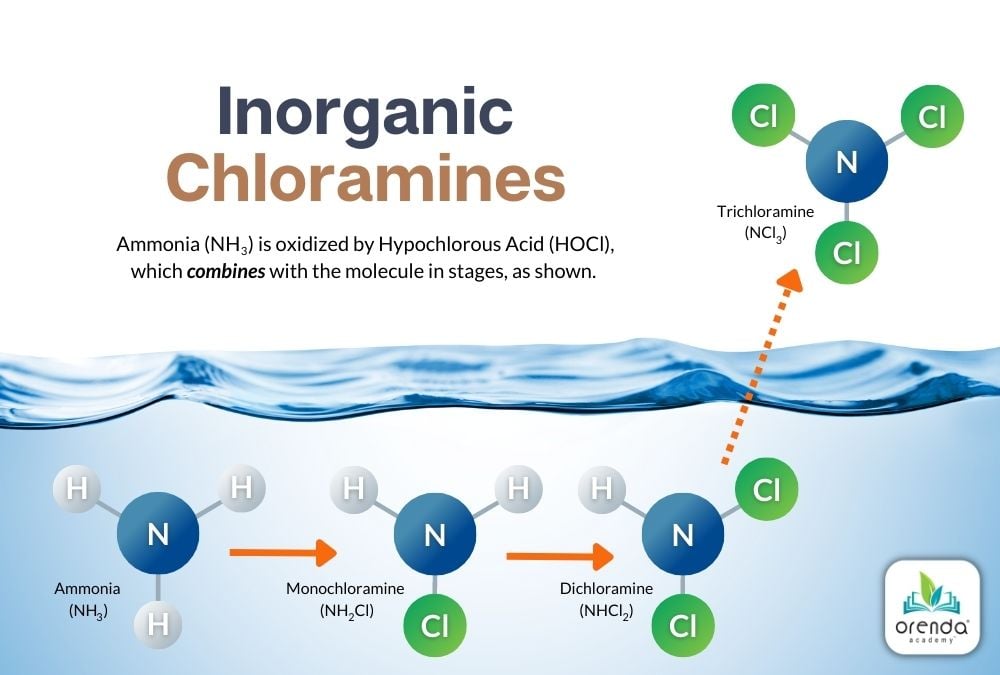
The chemical reaction that creates Monochloramine (NH2Cl) looks like this:
2NH3 + 2HOCl → 2NH2Cl + 2H2O
Ammonia + Hypochlorous Acid yields Monochloramine + Water
Notice that one of the three (3) Hydrogens in the ammonia was replaced by a Chloride (Cl). Further chlorination of monochloramine creates Dichloramine (NHCl2):
2NH2Cl + 2HOCl → 2NHCl2 + 2H2O
Monochloramine + Hypochlorous Acid yields Dichloramine + Water
Here again, a Chloride (Cl) has replaced one more Hydrogen. And, of course, even further chlorination yields the most noxious of chloramines that off-gas from pools, Nitrogen Trichloride, aka Trichloramine (NCl3):
NHCl2 + 3HOCl → NCl3 + 3H2O
Dichloramine + Hypochlorous Acid yields Trichloramine + Water
Finally, all Hydrogens have been replaced by chlorides to create Nitrogen Trichloride (trichloramine).
Chloramines are weak disinfectants
Referring back to the breakpoint chlorination graph above, the curve rises from point (A) to (B). This happens as chloramines are forming. The curve rises because chloramines––believe it or not––have some disinfection capability. They count toward Total Available Chlorine (TAC), which can be read on a total chlorine test.
Chloramines are much slower and weaker than the active, killing form of free chlorine, Hypochlorous Acid (HOCl). However, their slower reactivity can be considered an advantage in some circumstances. Many drinking water treatment plants deliberately add ammonia to the water to create chloramines.2 This helps keep the water disinfected for longer as it travels through the water grid.
Eventually, as more chlorine is added, it destroys the very chloramines byproducts it was creating. This begins at threshold (B) on the graph. This is why the line curves down toward point (C).
Chlorine continues to destroy until chloramines cannot be oxidized any further. After this threshold, a free chlorine residual can build. This threshold (C) is called the breakpoint.
Beyond the breakpoint
Breakpoint chlorination is not a single moment in time. Rather, it is a continual, ongoing process. For water quality and disinfection purposes, it's important for swimming pool chlorination to stay ahead of the breakpoint. When chlorine falls behind, superchlorination may be required to get ahead of the breakpoint again.3
Only after the oxidant demand has been addressed can disinfection occur. Therefore, only after breakpoint chlorination has been exceeded can a residual of free chlorine build. Until that point, chlorine has its hands full, oxidizing its way to the breakpoint and beyond.
Beyond the breakpoint, free chlorine residual builds. If there are leftover byproducts that cannot be further oxidized, they remain as combined chlorine. The sum of both free and combined chlorine is total chlorine.
FC + CC = TAC
Free chlorine + combined chlorine = total available chlorine
Superchlorination
Superchlorination, or shocking, is adding a high dose of chlorine at once. The term is often used interchangeably with breakpoint chlorination, but that's technically inaccurate. If the combined chlorine level in the water gets above an acceptable level (0.2 ppm in most places), the pool may require superchlorination to destroy the chloramines, exceed breakpoint, and build a free chlorine residual again. Shocking is the most widely-used method of reducing combined chlorine levels.
The conventional wisdom in the pool business is a shock of 10x your combined chlorine level in additional free chlorine. However, according to renowned chemist Richard Falk, the 10x number is more than needed.
"The molar ratio of chlorine to ammonia is 1.5:1 or 3:2, but since ammonia is measured in ppm N units while chlorine is measured in ppm Cl2 units, with the factor of 5.06 difference this is a ppm ratio of 7.6 to 1. Because forming dichloramine requires 2 moles of chlorine for 1 mole of ammonia and because of side reactions that can occur, the actual chlorine to ammonia ppm ratio is around 8-10x which is where the 10x rule came from. However, this is wrong since CC is in ppm Cl2 units (so no factor of 5.06) and monochloramine already has 1 of the 1.5 chlorine attached to it already. To oxidize monochloramine, it takes from 0.5 to 1.0 times the CC level. Even if the CC were urea, it takes 2-3 times the CC level, not 10x. Of course, the higher the FC level the faster reactions occur, but there is no magic 10x amount." - Richard Falk3
Breakpoint is hard to achieve when the overall oxidant demand exceeds the chlorine available to handle it. The oxidant demand in these cases can be metals, nitrogen compounds, non-living organics, or any combination of them.
If you’re shocking your pool frequently to reach breakpoint chlorination, ask yourself how you got there. Clearly, the normal chlorine levels in your pool are not enough to meet the demand. So think about how the demand itself got there. The fact is, if a swimming pool has ANY combined chlorine in it, there are nitrogen compounds in the water. Nitrogen is what chlorine combines with. So find the sources of nitrogen and address them directly (if possible).
Nitrogen sources
In commercial swimming pools, the most common nitrogen sources we find are cleaning chemicals that contain ammonia, floor cleaning products, and the bather load itself.4
In residential swimming pools, algaecides are the most common nitrogen source we see. Anything with a variant of the word "ammonia" in it will be nitrogen-based. Think ammonium, ammonium sulfate, dimethyl ammonium chloride, etc.. Look out for the word "quat" too. Quat is short for quaternary ammonia.
Bathers introduce urea from urine and sweat. In our experience, nitrogen-based chemicals can increase combined chlorine much more than bathers alone can. It would take a lot of swimmers peeing to match an algaecide or deck cleaner getting into the pool gutter. It's best to avoid any and all chemicals that contain nitrogen/ammonia of any kind. Prevention is easier than remediation.
Conclusion
Breakpoint chlorination is a threshold that must be exceeded for a free chlorine residual to build. It only includes nitrogen compounds, since chlorine combines with nitrogen compounds to create combined chlorine. Combined chlorine is total chlorine minus free chlorine, so it can be calculated from chlorine test results.
Superchlorination is not the only way to handle combined chlorine. There are secondary systems like UV, Ozone, and AOP that supplement chlorine well. Enzymes can also be used to break down carbon bonds and simplify organic nitrogen compounds into inorganic ammonia.
We favor a minimalist approach. Chlorine is a phenomenal disinfectant, but a relatively weak oxidizer. If we can assist chlorine in removing nitrogen compounds––either from prevention or destruction––water quality improves.
1 The breakpoint is when chlorine has oxidized combined chlorine compounds to a point where they cannot be oxidized further. These combined chlorine compounds are generically referred to as "chloramines", but technically chloramines are just inorganic ammonia combined with chlorine. In reality, any and all nitrogen compounds will combine with chlorine when being oxidized. They create countless variations of disinfection byproducts (DBPs).
2 US EPA. (Updated March 2023). Chloramines in Drinking Water. Drinking Water Requirements for States and Public Water Systems.
3 This post was originally on the Pool Genius Network, but the page has since been taken down. At the time of originally publishing this article, it was still online.
4 Bathers introduce urine and sweat, which contain urea. Urea is an organic nitrogen compound, meaning it contains carbon. This makes it more complex, meaning more chlorine is required to oxidize and remove it. This also yields more byproducts than inorganic ammonia.
5 jwpeifjwpe