Pool Sanitizers, Part 1: Chlorine

Chlorine is the most popular sanitizer/disinfectant used in swimming pools. This article covers the different types of chlorine and how to use them. We will link out to more in-depth articles for each type of chlorine (eventually).
Covered in this article:
- Chlorine chemistry
- Types of pool chlorine
- Non-stabilized primary pool chlorine types
- Sodium Hypochlorite (liquid chlorine)
- Calcium Hypochlorite (cal hypo)
- Lithium Hypochlorite (lithium hypo)
- Saltwater Generated Chlorine (salt system, or SWG)
- Chlorine Gas (Cl2)
- Stabilized pool chlorine
- Dichloro-s-triazinetrione dihydrate (Dichlor)
- Trichloro-s-triazinetrione (Trichlor)
- Non-stabilized primary pool chlorine types
- Chlorine byproducts
- Salts, calcium and CYA
- Conclusion
Chlorine chemistry
Free chlorine in a swimming pool is essential for keeping the water clean and safe. After all, chlorine is the pool's first line of defense against germs and viruses. Despite advances in secondary disinfection technology, the need for a residual sanitizer remains. But the bulk of chlorine consumption is due not to the sanitizer demand, but to the oxidant demand, which we cover at length in Pillar 2. Now, before getting into each type of chlorine, let's establish something.
ALL chlorine types, when added to water, convert into two forms of Free Chlorine (FC):
- The strong, killing form of chlorine, Hypochlorous Acid (HOCl).
- The weaker, slower form of chlorine, Hypochlorite Ion (OCl-).
Cl2 + H2O → HOCl + HCl
Chlorine(gas) + Water → Hypochlorous acid + Hydrochloric acid
Then, a Hydrogen dissociation reaction occurs almost immediately within HOCl itself:
HOCl ⇌ H+ + OCl-
Hypochlorous acid ⇌ Hydrogen ion + Hypochlorite ion
This dissociation of Hydrogen from HOCl stays in equilibrium, as a function of pH. In other words, pH controls this equilibrium, and thus the strength of chlorine (%HOCl)–but only in pools without cyanuric acid (CYA)!
All chlorine types become HOCl and OCl-. Beyond that, the differences in chlorine chemistry are byproducts left behind and each chlorine type's temporary pH impact.
Types of pool chlorine
We are not listing all sanitizers–such as chlorine alternatives–in this article. We have another article about these alternatives. We are also not talking about secondary disinfection systems because they do not provide a residual sanitizer. Let's stay focused on different chlorine types.

- ⚠︎ Different chlorine types are volatile and hazardous if mixed together. Two types of chlorine together can spontaneously combust or even explode. NEVER store, handle, or mix different chlorines together.
- ⚠︎ Never mix any type of chlorine with acid directly. The fumes are toxic and the reaction can be flammable.
- Elemental chlorine is a halogen gas (Cl2). It is extremely strong and effective, but because of safety hazards, it is outlawed in most places. We mention it here for informational reasons, but it is not a common solution anymore.
- There are two categories of chlorine we will cover: non-stabilized and stabilized. The difference is cyanuric acid is blended into stabilized chlorine.
For more detailed chemistry info, we hyperlink to some of our online sources. Our primary resource for this chlorine information is the IPSSA Basic Training Manual, Chapters 5 and 6.
Non-stabilized primary chlorine types
These five types of chlorine contain no cyanuric acid: Liquid chlorine, cal hypo, lithium hypo, salt-generated chlorine, and chlorine gas.
1. Sodium Hypochlorite (Liquid Chlorine)
- Other names: Liquid Chlorine, Bleach
- Physical form: Liquid
- Chemical Formula: NaOCl
- Chemistry in water: NaOCl + H2O → HOCl + Na+ + OH-
- Trade %: sold in 10% and 12.5% strengths
- pH: ~13
Pros: Liquid chlorine is affordable, easy to measure, and easy to use. It is also easy to set up on chemical automation with a timed feed pump. As far as pool sanitizer goes, bleach is popular in both residential and commercial pools, and readily available in every pool market. As far as homeowners go, liquid chlorine is arguably the safest and most practical chlorine to use.
Cons: Liquid chlorine's shelf life is limited. The lower the active chlorine percentage, the longer the shelf life. Also, if liquid chlorine is spilled, it makes a mess and needs to be cleaned up according to the instructions on the Safety Data Sheet (SDS). Contrast that with a dry or granular chlorine which can be swept up and collected off a dry surface. The other downside to liquid chlorine, from the pool pro's perspective, is size and weight. A pickup truck can only hold so much liquid chlorine, because chemicals cannot be stacked in trucks. Liquid chlorine is heavy too (~10 pounds per gallon). Compared with a large bucket of 3" tabs, liquid chlorine takes up far more space in a truck bed.
How to use Liquid Chlorine
We will have a future article about how to manage a liquid chlorine pool, from a broader chemistry perspective. For now, just know that liquid chlorine can be measured and poured directly into the pool, though doing so in the evening is ideal (less sunlight contact time). We recommend pouring it either around the perimeter or above a return inlet to spread the chlorine around. Always use safety glasses and gloves when handling liquid chlorine. After pouring, close the cap of the bottle and dunk it into the water in case any chlorine spilled on the outside of the bottle. Store liquid chlorine in a well-ventilated area, not in direct sunlight, and nowhere near other types of chlorine.
You can also install liquid chlorine on a feed pump, similar to how we set up a feed pump for enzymes.
2. Calcium Hypochlorite (Cal Hypo)
- Other names: Cal Hypo, Shock
- Physical form: Dry granular, powder, or pressed tabs or briquettes (both rapid-dissolving and slow-dissolving)
- Chemical formula: Ca(ClO)2
- Chemistry in water: Ca(ClO)2 + H2O → HOCl + Ca+ + OH-
- 36.2% chlorine (by weight) in 73% cal hypo. 70% available chlorine.
- 33.7% chlorine (by weight) in 68% cal hypo. 65% available chlorine.
- pH: ~10.8 when dissolved in water
Pros: Cal Hypo is a powerful sanitizer and oxidizer that also leaves behind calcium in the water. Particularly for pools with plaster surfaces, cal hypo helps saturate the water with calcium to reduce the risk of etching. Measure calcium in the fill water and plug it into the an LSI dosing calculator. You will find that higher calcium not only increases the LSI, but also buffers the LSI because calcium is so steady. We at Orenda like calcium, but at some point you may need to dilute water to bring the level back to a manageable level. That's why LSI Balance and Calcium Management is our First Pillar of Proactive Pool Care.
Cons: Cal Hypo comes in the form of powder or pressed tabs/pucks or briquettes. These pressed cal hypo products require a specific feeder to be introduced to the water. These feeders tend to get clogged with calcium and organic waste buildup, and require cleaning every few weeks. The bigger issue with cal hypo, however, is its volatility. It is a strong oxidizer and fire hazard, and when it is near certain chemicals (other types of chlorine, for example), it can spontaneously combust. Never, and we repeat, never put any other type of chlorine in a cal hypo feeder, or cal hypo in any other feeder.
Warning: Even introducing cal hypo directly to organics (body oils, ammonia, human sweat) can cause a fire. Add chlorine to water, not water to chlorine. Always use gloves and protective equipment when handling it, and keep it safely stored.
How to use Cal Hypo
We will have a future article to explain cal hypo pool management. For now, cal hypo granular shock should be used according to the label. Pre-dissolve in a bucket of water and pour around the perimeter of the pool. Pro tip: reduce pH with diluted acid in a bucket prior to rinsing the bucket and dissolving cal hypo. Adding cal hypo without reducing pH ahead of time can lead to clouding the water. Be sure to thoroughly rinse the bucket before and after mixing cal hypo! It's vapors are strong and can be harmful if you inhale them too much.
As for cal hypo feeders, only use the brand-specific cal hypo product that matches the feeder. Consult chlorinator manufacturers for instructions on how to operate. No matter what, NEVER put cal hypo in a trichlor feeder or allow it to come into contact with any other type of chlorine.
A recent product in the pool business is the non-stabilized tab (NST), which is a slower-dissolving cal hypo tablet. It is designed to be put in the skimmer. This is the only skimmer-approved chlorine that we know of. Consult the manufacturer for instructions.
3. Lithium Hypochlorite (Lithium Hypo)
- Other names: Lithium hypo
- Physical form: Dry granular
- Chemical formula: LiOCl
- Chemistry in water: LiOCl + H2O → HOCl + Li+ + OH-
- 35% available chlorine (by weight).
- pH: ~10.8 when dissolved in water
Pros: Lithium hypochlorite is a stable form of chlorine with a longer shelf life than liquid bleach or cal hypo. It is not a fire hazard, and also boosts total alkalinity when added to the water, as well as raising the pH. Lithium hypo is fast-dissolving and can be introduced as a powder, or pre-dissolved in a bucket before introducing as a liquid.
Cons: Lithium hypochlorite is rarely used, primarily because of cost, and its relatively low strength. For residential pools it may be adequate, but it is generally regarded as an insufficient pool sanitizer to handle the demands of a busy commercial pool. Good luck finding it on the shelves.
How to use Lithium Hypo
Lithium hypochlorite is used much in the same way as calcium hypochlorite. Use with caution, and pre-dissolve before adding to a pool.
4. Saltwater Generated Chlorine (SWG)
- Other names: Salt pool, saltwater pool, salt system, salt cell
- Physical form: N/A
- Chemistry in water: 2NaCl + 2H2O →⚡︎→ Cl2⇡ + 2NaOH + H2⇡
- pH: neutral (Chlorine gas neutralizes NaOH after the salt cell)
Yes, salt pools ARE chlorine pools. When salt dissolves in water, the sodium (Na+) separates from the chloride (Cl-). The chloride is then charged by electricity in a process called electrolysis. This process creates chlorine gas (Cl2⇡), sodium hydroxide (NaOH), and Hydrogen gas (H2⇡). The Chlorine gas is acidic when it dissolves in water, creating HOCl and HCl:
Cl2 + H2O → HOCl + HCl
Chlorine gas + water → Hypochlorous acid + Hydrochloric acid
The byproduct sodium hydroxide (NaOH) has a very high pH (13+). Chlorine gas and sodium hydroxide neutralize each other's pH, creating an almost perfectly-neutral pH chlorine. However, anyone who has operated a saltwater pool knows that the pH tends to rise quickly. This is actually due to the turbulence created by the Hydrogen gas bubbles (H2⇡), which encourages the loss of carbon dioxide (CO2). And as we discuss in detail in other articles, the loss of CO2 raises the pH of water.
Pros: Relatively constant feeding of chlorine as long as the pool's circulation system is on, and the water temperature is warm enough. Also, after the equipment is installed, chemical costs are very low (with the exception of constantly having to lower the pH with acid or injected CO2).
Cons: Salt chlorine generators are unable to produce more pool sanitizer if demand increases, which may require users to supplement chlorine as needed (shock the pool). Also, if the pool is not electrically bonded properly, electrochemical corrosion can occur quickly. And since salt cells produce heat and sodium hydroxide, the LSI in the salt generator chamber is very high. That high LSI invites carbonate scale to form, which then fractures off when the cell's electrical current reverses polarity (changes direction), causing calcium flakes in saltwater pools.
The other con to saltwater is the total dissolved solids are high...generally 3,000 on the low end. Higher TDS leads to cloudy water, and as water evaporates out during those hot, sunny days, the salt stays behind.
But scale on salt cells can be mitigated with good LSI management, having about 60 ppm TA to reduce the pH ceiling, and chelating calcium with SC-1000.
How to use salt generated chlorine
Follow manufacturer's instructions for the salt chlorine generator, and keep the system clean and maintained. Maintain the desired ppm of salt in the water, and select the chlorine output you want. We recommend not exceeding 70-80% output. Usually 40% is appropriate.
5. Chlorine Gas
- Other names: elemental chlorine
- Physical form: Gas
- Chemical formula: Cl2⇡
- Chemistry in water: Cl2 + H2O → HOCl + HCl
- Available chlorine by volume: 100%
- pH: <1.0 when dissolved in water
Pros: Chlorine gas is pure and fast-acting. If you can even use it anymore, it's generally cheap and can be added quickly to water.
Cons: Chlorine gas is hazardous and therefore outlawed in most places. It's hard to find, and even if you can, it takes special equipment and transportation precautions to use it. Injecting chlorine gas is harder than simply pouring in a product. The other negative of gas chlorine is it's acidity. It will drop pH and alkalinity every time you use it.
How to use Gas Chlorine
Gas chlorine is injected into solution. In commercial pools, it is tapped into the plumbing much like an ozone or CO2 injector system would be. We hardly ever see gas chlorine anymore, due to its volatility. For residential pools, an injector can connect to a standard cleaning pole, which is submerged into the water as gas flows into the pool. But again, almost nobody uses gas chlorine anymore. Ahh, the good ole days of using dangerous chemicals without much understanding of them. Nostalgia.
Stabilized chlorine types
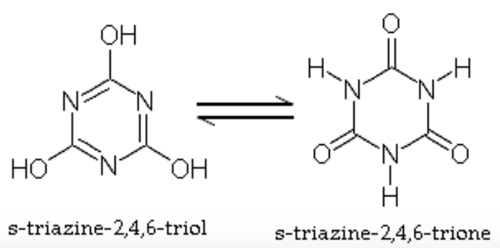
6. Sodium Dichloro-S-Triazinetrione dihydrate (Dichlor)
- Other names: Sodium dichlor, dichlor shock
- Physical form: Dry granular
- Chemical formula: NaCl2C3N3O3
- Chemistry in water: NaCl2C3N3O3 + 2H2O → 2HOCl + NaH2C3N3O3
- 27.7% chlorine (by weight). 54.8% available chlorine.
- pH: ~6.5 when dissolved in water
Pros: Neutral pH, long shelf life, fast dissolving. Dichlor is commonly used as a shock and a startup chlorine to introduce some cyanuric acid to the water...but not too much. It is great for fiberglass and painted pools because the pH is neutral.
Cons: Dichlor is a fire hazard, and is not easily introduced via an automated feeder system due to its fast-dissolving nature. It adds cyanuric acid to the water, which is good up to a certain point...but continual use of dichlor will keep increasing the levels of stabilizer in the pool. It dramatically slows down chlorine, and eventually can become a problem.
How to use Dichlor
Dichlor is a granular shock that can be directly added to the pool, but we strongly recommend pre-dissolving it in a bucket of water and pouring around the perimeter of the pool. Always use safety glasses and gloves.
7. Trichloro-S-Triazinetrione (Trichlor)
- Physical form: Pressed tabs (most commonly 3") or dry granular
- Chemical formula: Cl3C3N3O3
- Chemistry in water: Cl3C3N3O3 + 3H2O → 3HOCl + H3C3N3O3
- 45.8% chlorine (by weight). 90.6% available chlorine (in 99% trichlor).
- pH: ~2.8 when dissolved in water
Trichlor was originally developed to sustain chlorine throughout the week as a supplement to liquid chlorine, because non-stabilized liquid chlorine could not last more than a few days in direct sunlight. Trichlor is about 55% CYA, which means every bit of the chlorine that is introduced is stabilized.
Unfortunately, trichlor has become a primary chlorine because of its convenience. While trichlor has its benefits as a supplemental chlorine, it is not a primary chlorine. It is primarily a CYA delivery product...that just happens to chlorinate too. Too much trichlor use leads to overstabilization and other problems.
Pros: Trichlor is affordable and slow-dissolving. It is popular for year-round residential pools by putting tabs in an in-line feeder or a floater. Sometimes 3" trichlor tabs are placed in the skimmer baskets, but this is a bad practice. The main benefits of trichlor are cost, ease of handling and transport (a large bucket has a small footprint in a pickup truck compared to cases of liquid chlorine), and the fact that trichlor will continue to give a pool a free chlorine reading after a week. The other benefit, in a way, is trichlor's acidic pH, because it suppresses the pH from rising naturally during the week.
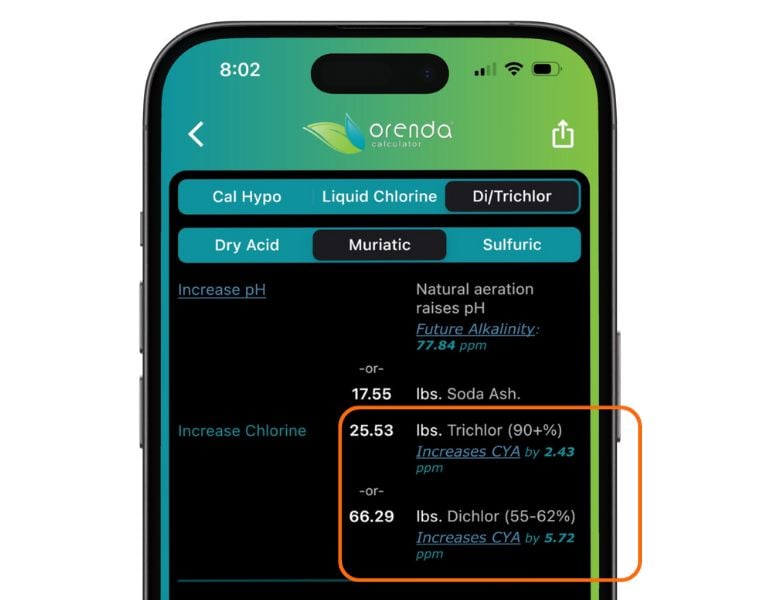
Cons: Trichlor is 55% cyanuric acid, and it introduces 6-6.5 ppm of CYA per pound per 10,000 gallons of water. The CYA accumulation occurs quickly and high CYA overstabilization is the largest problem with trichlor. High CYA not only makes the LSI of the water lower (aggressive), it also dramatically slows chlorine's killing speed. Minimal CYA is the Fourth Pillar of the Orenda program, and regular use of trichlor simply does not fit into our philosophy. Trichlor is also acidic, so tab floaters can etch/fade pool surfaces, and tabs in the skimmer can corrode pool equipment (like heaters).
How to use Trichlor
 Trichlor tabs belong in a dedicated in-line trichlor feeder. The other alternative is putting them in a trichlor floater. Never put trichlor tabs in the skimmer, or in any other type of chlorinator.
Trichlor tabs belong in a dedicated in-line trichlor feeder. The other alternative is putting them in a trichlor floater. Never put trichlor tabs in the skimmer, or in any other type of chlorinator.
Chlorine byproducts
Each type of chlorine turns into HOCl and OCl-, but has different byproducts left behind. We have mentioned some of them in each product listing, but there here is a wrap-up overview of them.
Salts, Calcium and CYA
Every chlorine leaves chlorides behind, which means they increase the salt levels in the water.1 Salt will not accumulate in a saltwater pool, however, because the chlorine gets regenerated in the salt cell, and the cycle repeats itself.
The three main types of chlorine that we add to pools, however, leave byproducts behind that increase TDS over time.
- Sodium hypochlorite leaves behind sodium and chlorides (salt),
- Calcium hypochlorite leaves behind calcium, and
- Dichlor/Trichlor leave behind cyanuric acid (CYA).
To know exactly how much your chlorine will be adding based on the amount you are adding, we have added these into the Orenda Calculator™.
Conclusion
While there are several types of chlorine products, chlorine chemistry once it's dissolved in water is basically identical. The only differences between the products is what they leave behind in water. All of them introduce Hypochlorous Acid (HOCl) and its weaker counterpart Hypochlorite ion (OCl-).
Choosing the right chlorine type for you depends on your tap water chemistry and the needs of your swimming pool.
1 Salt, or sodium chloride (NaCl), separates when it is dissolved in water. Not every chlorine contains sodium, but every chlorine type will be reduced to chlorides (Cl-) after oxidizing or killing contaminants. That chloride will find available sodium ions (Na+) in the water. There is plenty of sodium in water, thanks to other products like sodium bicarbonate, soda ash, and of course, sodium hypochlorite.
