What are Chloramines?

Technically speaking, chloramines are chemical byproducts of chlorine oxidizing inorganic ammonia in water. The term chloramines is used generically to describe all disinfection byproducts (DBPs) that result from chlorine oxidizing nitrogen compounds. Chloramines and these other DBPs are the main cause of air quality problems in indoor pools.
Covered in this article:
- Chloramine formation
- Bather comfort
- Indoor air quality (IAQ) problems
- Chloramine destruction
- Chemicals that reduce combined chlorine
- Systems that reduce combined chlorine
- Chloramine prevention strategy
The widespread use of the term "chloramines" can sometimes lead to confusion. Let's be specific about the terms we will use in this article because some terms are used interchangeably.
- Chloramines are only three specific byproducts that form from chlorine oxidizing inorganic ammonia. They are monochloramine, dichloramine, and trichloramine.
- Combined chlorine refers to any and all nitrogen compounds that combine with chlorine as part of the oxidation process. There are countless variations and oxidation states of these compounds.1 Combined chlorine, therefore, is a broader term that we will use more frequently in this article.
- Oxidant demand refers to all non-living contaminants in water. There are three main categories of oxidants in swimming pools: metals, nitrogen compounds, and non-living organics.
We invite you to further research DBPs if you are interested in this topic. This article is just an overview.
Chloramine formation
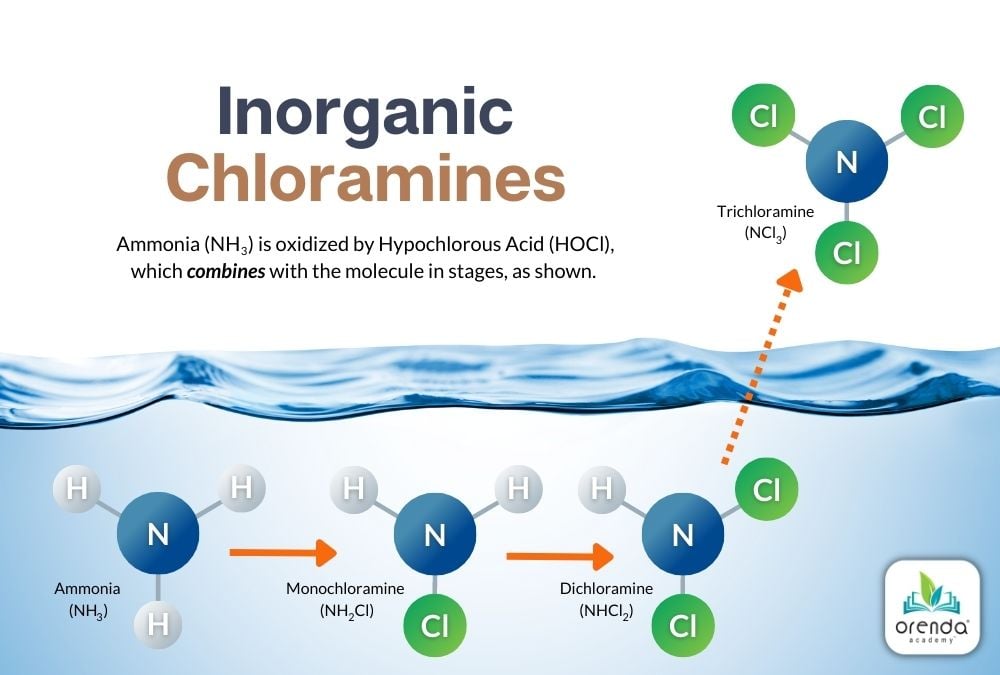
Inorganic chloramines occur when the active form of chlorine, Hypochlorous Acid (HOCl), oxidizes ammonia (NH3). Above, you see a graphic of the sequence of chlorine gradually replacing the Hydrogens that once belonged to ammonia. Eventually, it becomes a Nitrogen with three chlorides, called nitrogen trichloride (aka trichloramine). Let's break down each of these reactions.
Monochloramine (NH2Cl)
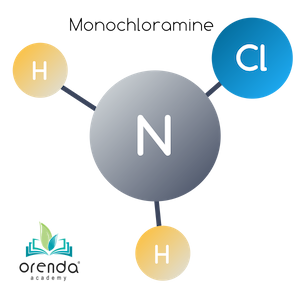
When there is a five-to-one (5:1) weight ratio (1:1 molar ratio) of HOCl to NH3, one of ammonia's Hydrogen ions is replaced by a Chloride ion. This reaction occurs quickly at normal swimming temperatures. The colder the water, the slower the reaction, and vice versa. The product of this reaction is monochloramine (NH2Cl).
HOCl + NH3 → NH2Cl + H2O
Hypochlorous Acid + Ammonia → Monochloramine + Water
Dichloramine (NHCl2)
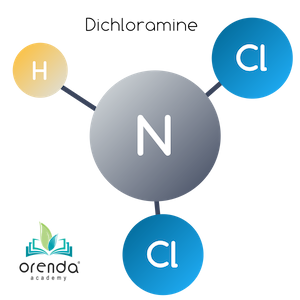
Oxidation continues against monochloramine until an additional 5:1 weight ratio threshold is met. At this point, another Hydrogen is replaced by a Chloride (Cl) ion. Now Dichloramine (NHCl2) has been formed. If you keep count, this is now a 10:1 weight ratio of HOCl to what was once Ammonia. The reaction looks like this:
HOCl + NH2Cl → NHCl2 + H2O
Hypochlorous Acid + Monochloramine → Dichloramine + Water
Trichloramine (NCl3)
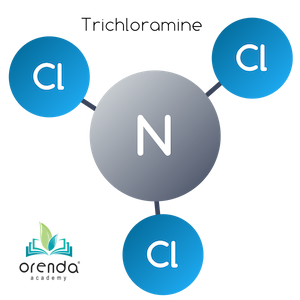
When another HOCl reaches another 5:1 weight ratio with Ammonia––now a total weight ratio of 15:1 (3:1 molar ratio)––the last Hydrogen is replaced by a third chloride ion. Trichloramine (NCl3) is formed.2
HOCl + NHCl2 → NCl3 + H2O
Hypochlorous Acid + Dichloramine → Trichloramine + Water
Unlike monochloramines and dichloramines, trichloramine goes airborne. This off-gassing is an inevitable part of the process. Trichloramines are the primary culprit behind indoor air quality problems.
Related: History of Indoor Pool Air Quality (IAQ) Problems, Part 1
Bather comfort

A child with irritated eyes and face after playing in an indoor swimming pool. Photo used with permission.
The swimming pool industry has pH standards of 7.2 to 7.8, with an ideal pH of 7.4-7.6. One of the three justifications for this pH range is bather comfort, supposedly because the pH of our eyes and skin. We believe that notion to be debunked. That said, actual bather discomfort is due to these disinfection byproducts in the water and air.
Indoor air quality (IAQ) problems
Trichloramines and other off-gassing byproducts are known to be harmful irritants. Chloramine pollution is a widespread problem for indoor pools worldwide. Chloramines are not only harmful to people; they are also highly corrosive to metals and electronics. To learn more about chloramines and their impact on indoor air quality, start here.
Prolonged exposure in indoor pools can lead to long-term health consequences like upper respiratory disease and lung scarring.3 We know from personal experience. Swimmers often use inhalers just to get through practice each day. And until the air system and water systems are optimized for indoor air and water quality, the problems will persist.4
Chloramine destruction
Now that we know how chloramines are formed, let's discuss how to destroy them. To do this, we will name chemicals and systems that can be used to reduce combined chlorine. We will also discuss ways to address chloramines once they go airborne.
Chemicals that reduce combined chlorine
Chlorine
The most common way to address combined chlorine is by using more chlorine. The aim is to exceed a threshold called the breakpoint, after which a free chlorine residual can build. Staying ahead of this point is known as breakpoint chlorination.
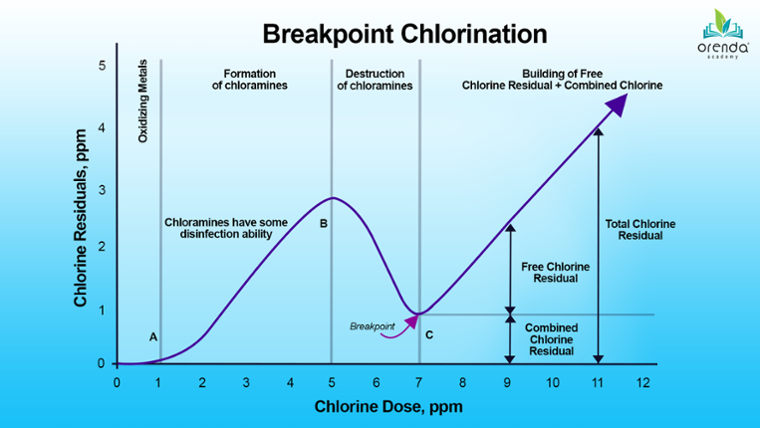
Breakpoint chlorination should not be confused with superchlorination, or shocking. The terms are often used interchangeably. Breakpoint chlorination is an ongoing process, whereas shocking is adding a heavy dose of chlorine at once to exceed the breakpoint once chlorine has fallen behind.
The logic behind shocking is to overwhelm the entire oxidant and sanitizer demand in the water, including partially burned-up contaminants and byproducts. Superchlorination is a surge of free chlorine to crush everything in its way.
There are a few issues with shocking as a strategy. First and foremost, it takes a lot of chlorine to shock a pool, and sometimes even more chemicals to restore balance afterward. For example, in public swimming pools, a chlorine neutralizer like sodium thiosulfate or SC-1000 might be required to bring chlorine back down to acceptable levels before reopening the pool for swimmers.
In our opinion, shocking should used for disinfection purposes––like a fecal incident or disease outbreak. Shocking for oxidation reasons means that normal chlorination cannot handle your bather load. If that's the case, we recommend proactively managing the bather load.
Enzymes
Speaking of proactive, enzymes are a great option for this. Enzymes can break down organic nitrogen compounds (like urea) by removing carbon. Enzymes do not remove nitrogen from water, but they can simplify organic nitrogen compounds into their inorganic components. That, in turn, makes it easier for chlorine to fully oxidize the contaminants.
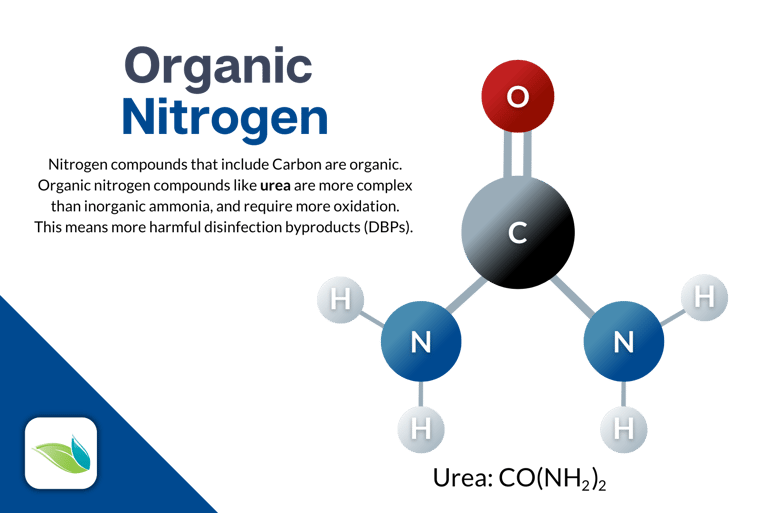
Non-chlorine shock
Non-chlorine shock products like potassium monopersulfate can also be used, even though it’s an oxidizer only, not a sanitizer. But potassium monopersulfate does not remove chloramines. It only helps prevent future chloramines by reducing organics and nitrogen precursors. In that sense, it is helpful and could be considered proactive.
Systems that reduce combined chlorine
Waterborne chloramines
Medium-pressure Ultra-Violet (UV) systems are an effective way to destroy formed chloramines that are still in the water.5 Low-pressure UV can destroy monochloramines, but it lacks the wavelengths to destroy dichloramine or trichloramine.
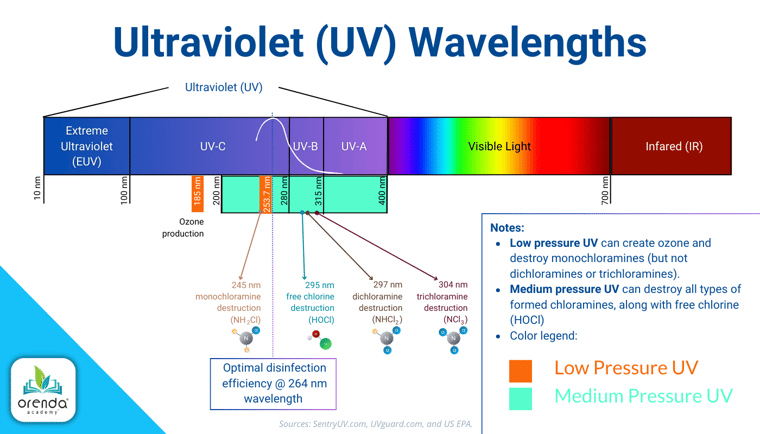
UV breaks apart formed chloramines, but it is not an oxidizer.6 That means UV cannot destroy nitrogen compounds until they have combined with chlorine. Another downside is that UV is a point-of-contact system that can only kill what it sees. There is no residual.
Two more secondary disinfection systems are Ozone (O3) and Advanced Oxidation Process (AOP). These systems are similar because they are incredibly powerful oxidizers that flow into circulation briefly. They can kill and oxidize just about anything in the water. Like UV, Ozone, and AOP are at the mercy of the pool's circulation. When set up and operated properly, these systems can destroy not only chloramines but also the nitrogen compounds that lead to their creation (like ammonia and urea). In fact, ozone is one of the most effective natural oxidizers and disinfectants on the planet.
Airborne Chloramines
Trichloramines and other DBPs destroy HVAC systems, metal structures (like a roof or structural steel), and metal pool equipment, such as starting blocks, lights or timing equipment.
There is only one system that we have seen to be truly effective at addressing airborne chloramines: The Paddock Evacuator®. It’s a source-capture ventilation system that targets and exhausts the harmful air in a pool and prevents HVAC systems from recirculating it.
Like every system, its effectiveness depends on certain variables in the natatorium. Indoor air quality has just as much to do with the shape of the room and its airflow design as it does the water chemistry. Fresh air does not happen by accident. It takes a coordinated strategy.
Chloramine Prevention Strategy
There is no 'magic bullet' to solve the chloramine problem in natatoriums. There are a multitude of factors to address. For natatoriums, the healthiest pool environment usually has:
- Adequate chlorination (minimal CYA on outdoor pools)
- Secondary disinfection system(s)
- Enzymes to reduce non-living organics and oils
- Pool turnover rates faster than 4 hours
- Chemical automation
- LSI balance
- Properly sized pool dehumidification system (indoor pools)
- Source-capture exhaust (indoor pools)
- Airflow design that allows for chloramine removal and fresh air recirculation
While chloramines in the water are an inevitable part of pool chemistry, they can be managed proactively. If you need help with chloramine issues on an indoor pool, reach out to us.
1 Chowdhury, S., Alhooshani, K., & Karanfil, T. (2014). Disinfection byproducts in swimming pool: Occurrences, implications and future needs. Water Research, 53, 68-109. https://doi.org/10.1016/j.watres.2014.01.017
2 The technical name for NCl3 is nitrogen trichloride, though that name is used interchangeably with trichloramine.
3 Rose, C. S., Martyny, J. W., Newman, L. S., Milton, D. K., Beebe, J. L., McCammon, J. B., Hoffman, R. E., & Kreiss, K. (1998). "Lifeguard lung": Endemic granulomatous pneumonitis in an indoor swimming pool. American Journal of Public Health, 88 (12), 1795-1800. https://doi.org/10.2105/ajph.88.12.1795
4 If you know of an indoor pool that struggles with the "pool smell", swimmers coughing, and corrosion on metal surfaces and equipment, the first step to solving the issue is first getting it diagnosed with an in-person facility evaluation.
5 Monochloramine and dichloramine stay in the water until they are either destroyed by UV or ozone, or combine with more chlorine to produce trichloramine. Trichloramine stays in the water for a short amount of time before off-gassing into the air, at which case UV and other water treatment systems become irrelevant. All secondary disinfection/oxidation systems (UV, Ozone, AOP) are at the mercy of the circulation rate, because they are point-of-contact systems that do not create a residual in the water.
6 Weng, S., Blatchely, E.R. (2013). Ultraviolet-Induced Effects on Chloramine and Cyanogen Chloride Formation from Chlorination of Amino Acids. Environmental Science & Technology. 47 (9), 4269-4276
DOI: 10.1021/es400273w